
MICROCHIP CAPILLARY ELECTROPHORESIS DEVICE FOR
AMPEROMETRIC DETECTION OF DNA WITH REDOX
INTERCALATION
Rohit Chand, You-Cheol Jang, Sandeep Kumar Jha
Dept. of Nanoscience and Engineering, Myongji University, Yongin, 449728, Republic of Korea
Yong-Sang Kim
Dept. of Nanoscience and Engineering, Dept. of Electrical Engineering, Myongji University
Yongin, 449728, Republic of Korea
Keywords: Capillary electrophoresis, Genomic DNA, Methylene Blue, Amperometry.
Abstract: Microfabricated biochips are very efficient platforms for analysis of biologically important molecules such
as DNA, RNA, enzymes, antibodies etc. These devices requires sample in micro/nano volume and produces
faster and better results. For these reasons, we fabricated a simple, disposable microfluidic device for
amperometric detection of DNA intercalated with methylene blue redox dye. The devices were fabricated
using conventional photolithographic method. The microchannels were laid in PDMS using negative
molding. The microchannel was 2 cm in length while the height and thickness were 250 µm and 200 µm
respectively. The electrodes used for electrophoretic separation and amperometric detection were made of
gold and were deposited by thermal evaporation on glass substrate. For the detection of DNA, fish sperm
DNA was intercalated with methylene blue as an analyte. The cyclic voltammograms of free methylene blue
and those of different concentrations of DNA intercalated with same amount of methylene blue was
obtained in this study. The intercalated DNA was then injected in the sample reservoir of fabricated device
and subjected to a separation electric field. The i-t curve was monitored for this process. The
electropherograms thus obtained suggested a possibility of rapid detection of DNA with high sensitivity and
reproducibility.
1 INTRODUCTION
Ever since the publication of DNA’s double helix
structure, electrophoresis has been a standard,
indispensible analytical tool in modern biochemistry
and molecular biology; electrophoretic procedures
are used in almost every aspect of basic or applied
biomedical and clinical research.
Traditional techniques as performed today in the
majority of laboratories, is still typically a manual
process which makes electrophoretic procedures
often time consuming and labour intensive.
Capillary electrophoresis (CE) on the other hand, is
a relatively new separation technique that is ideally
suited for handling small amounts of sample
material. The advent of microfabricated fluidic
devices in the past decade promises to address some
of these issues by miniaturizing and automate these
devices including the CE process (In-Je Yi, 2006).
More recently, electrochemical detection (ED)
has been reported for microchip (MC) CE (Ju-Ho
Kim, 2004). This mode of detection is ideally suited
for miniaturization to the microchip format. If the
power supply and electrochemical analysers are also
miniaturized, it is possible to envision a complete
µTAS (Dolnik, 2000; Woolley, 1998).
A typical MC-CE device has channel widths
varying from 50 to 200 µm with typical straight
separation channels between 1 and 5 cm in length. A
serpentine or semi-circular design can be
implemented to increase the separation channel
length up to 15 cm (Jacobson, 1994; Culbertson,
2000).
DNA-binding or intercalating dyes have been
284
Chand R., Jang Y., Jha S. and Kim Y..
MICROCHIP CAPILLARY ELECTROPHORESIS DEVICE FOR AMPEROMETRIC DETECTION OF DNA WITH REDOX INTERCALATION.
DOI: 10.5220/0003290802840287
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 284-287
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
used for fluorometric and amperometric DNA assays
and in flow cytometry applications. Ethidium
bromide (EtBr) was the first of such intercalators to
be used for DNA assays. Interestingly, the resolution
of dsDNA separations in CE can be improved by
using intercalating dyes. This is usually done by
adding dye to the running buffer (and/or sample) in
concentrations of 0.5 to 5 mg/mL. The dye molecule
inserts itself (“intercalates”) between the base pairs
of DNA, changing the molecular persistence length,
conformation, and charge of the DNA.
Amperometric CE detection was first reported as
a detection technique for CE by Wallingford and
Ewing in 1987 for the quantitation of catechol and
catecholamines. Amperometric detection is based on
electron transfer to or from the analyte of interest at
an electrode surface that is under the influence of an
applied DC voltage. The result of electron transfer is
a redox reaction at the electrode that produces a
current that is directly related to the analyte
concentration. Thus, by analysing the DNA
intercalated redox-active dye by amperometric
method; it should be possible to analyse the
concentration of DNA after its capillary
electrophoretic separation. In the present work, we
fabricated a CE-AD device for CE separation
followed by amperometric analysis of DNA-
intercalated methylene blue (MB) dye, thus
providing the basis for detection of DNA fragments
by indirect means.
2 MATERIALS AND METHOD
2.1 Reagents and chemicals
DNA Sodium salt fish sperm (Ultra-Pure) was
obtained from Bio Basic Inc., Korea. Methylene
blue (MB) (Reagent grade) was purchased from
Biopure, Canada. PDMS Sylgard 184 was from Dow
Corning Corp. (Midland, MI, USA). SU-8 2000
negative photoresist and XP SU-8 developer were
from Micro-Chem Co. USA and AZ 1512 positive
photoresist and AZ developer was from AZ
electronics material, Korea. The other chemicals of
ACS grade were purchased from Sigma-Aldrich,
Korea. All solutions were prepared afresh, stock
solution were made using double-distilled deionized
water (DI) and further diluted to required
concentration using the supporting electrolyte.
2.2 DNA Precipitation
Fish sperm DNA obtained was precipitated using
ethanol precipitation. After precipitation, the
mixtures were then centrifuged to collect the
precipitate. The pellet was washed twice with 1 ml
of 70 % Ethanol. The pellet was air dried. Later, the
pellet was dissolved in 800 µl of DI water and used
for spectrophotometric study. For electrochemical
study the samples were further diluted with 200 µl
of 1 M KCl.
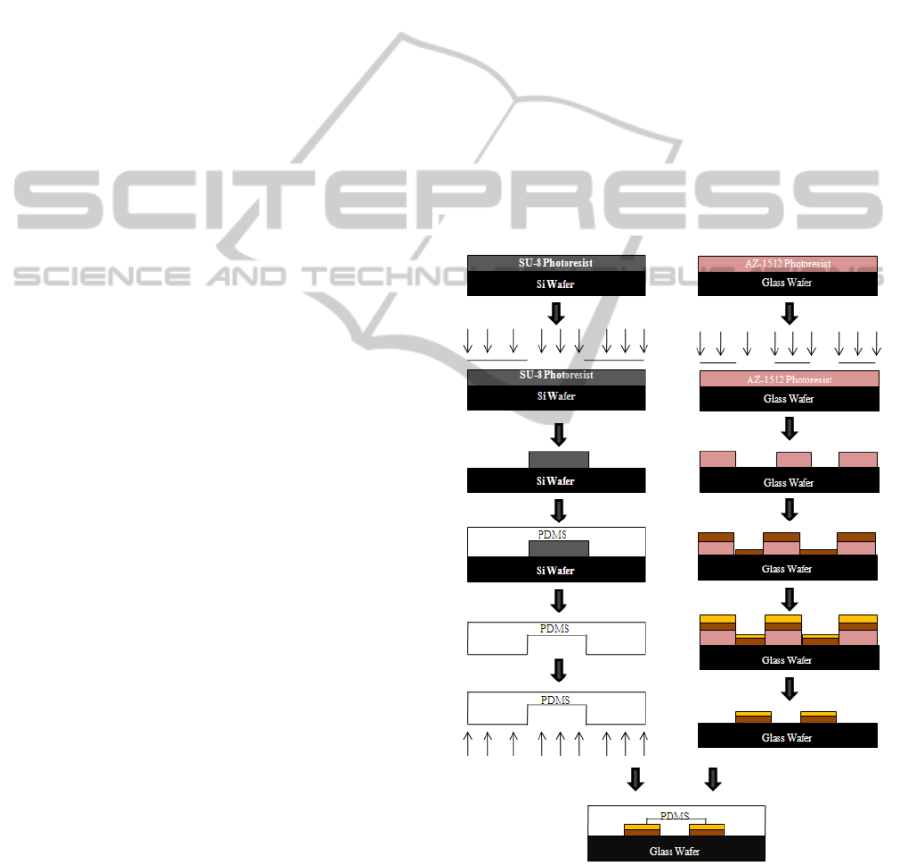
2.3 Fabrication of Microchip
A three-electrode detection system was used for CE-
AD. The simple process flow for the fabrication of
the CE-AD device is shown in Figure 1. We can
divide the procedure in two parts: fabrication of
microchannel in PDMS mold and laying gold
electrodes on glass substrate. The electrodes were
200 µM each in width. Gold electrode was choice
for the detection and separation electrodes due to its
inertness to redox reaction. The microchannels, each
Figure 1: Fabrication of CE-AD device.
200 µm in width, 250 µm in height and 2 cm in
length were fabricated using negative molding
method (Gi-Sung Joo, 2009). Finally the PDMS was
bonded on glass wafer using UV ozone bonding.
MICROCHIP CAPILLARY ELECTROPHORESIS DEVICE FOR AMPEROMETRIC DETECTION OF DNA WITH
REDOX INTERCALATION
285
2.4 Electrochemical Detection
Electrochemical detection was performed using
Electrochemical analyser, CHI 800B (CH
Instruments, USA). The three-electrode
electrochemical system was used for cyclic
voltammetry, which consisted of an Ag/AgCl
reference electrode (RE-5B, BASi), a Platinum wire
counter electrode (CHI 115) and a gold working
electrode (CHI101). Prior to voltammetry, the gold
and platinum electrodes were cleaned using chromic
acid, polished using electrode polishing kit (CHI
120) and cyclic sweep was performed in the range of
2 V to -2 V at a scan rate of 100 mV/sec in 0.1 M
Sulphuric acid until a stable curve was obtained.
Voltammetric sweep in sulphuric acid was repeated
before every voltammetric study. Cyclic
voltammograms of 200 mM Potassium chloride, 100
mM MB in 200 mM KCl, intercalated MB-DNA
complex sample and the other two precipitated
negative control were recorded at various potential
range and scan rate.
3 RESULTS AND DISCUSSION
Spectrophotometric study of MB-DNA complex
revealed that approximately 0.03 µM of MB binds to
1 mg/mL. Further electrochemical studies were
carried out with the free MB and MB-DNA
complex.
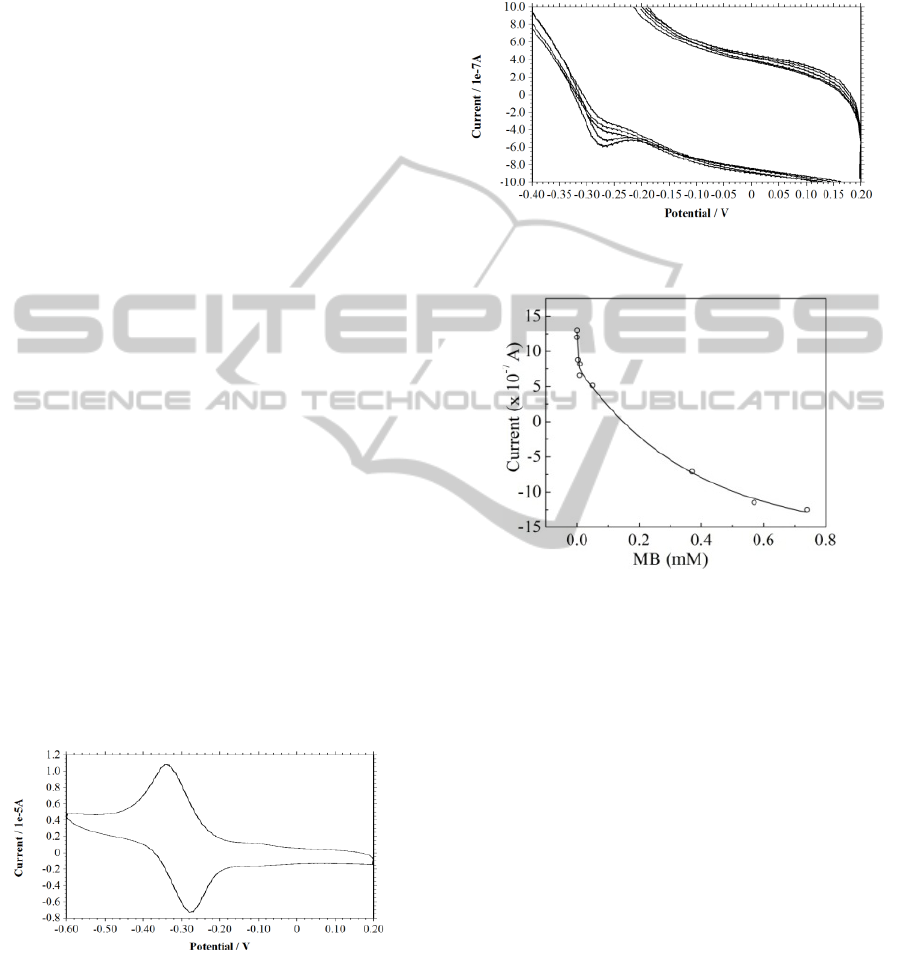
3.1 Electrochemistry of MB and DNA
A reversible redox cycle was obtained at 100 µM
concentration (Figure 2) in scan range 0.2 V to -0.6
Figure 2: CV of methylene blue, Conc.: 100 µM, in KCl
200 mM, scan rate 0.1 V/s.
V with scan rate 100 mV/sec. The cyclic
voltammograms shows a cathodic process of MB
(Ep
C
) at -0.280 V.The precipitated MB-DNA
complex was studied in the same range as that of
free MB. Figure 3 shows the cyclic voltammograms
of different concentration of intercalated MB-DNA
complex. The cyclic voltammograms of the complex
shows the similar redox process as that of free MB
with a cathodic process (Ep
C
) at -0.28 V.
Figure 3: CV showing peaks for different concentrations
of MB-DNA complex in 200 mM KCl, Scan rate: 0.1 V/s.
Figure 4: Shows a correlation between various
concentration of intercalated MB and peak current
produced by it using cyclic voltammetry. Suitable controls
without DNA and without MB did not produce any
corresponding peak, suggesting that the peak obtained in
the DNA-MB complex is only due to the MB bound to
DNA and not due to any other free MB.
3.2 Microchip CE-AD
Figure 5 shows the image of microchip CE-AD
device. The microchannel in the device was filled
with 200 mM KCl as a separation medium and
support analyte. A blank i-t curve was observed
without addition of any test analyte for the control
purpose. The i-t curve of free MB was also
monitored which showed no peak as free MB is
positive in charge and will not migrate in the
channel along the separation field for DNA.
The electrophoretic separation for MB-DNA
complex was initiated by adding 2 µL of complex
into the sample reservoir and a separation voltage of
100 V was applied. DNA-MB complex as migrating
towards anode is detected amperometrically using
in-channel gold working electrode. Figure 6 shows
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
286
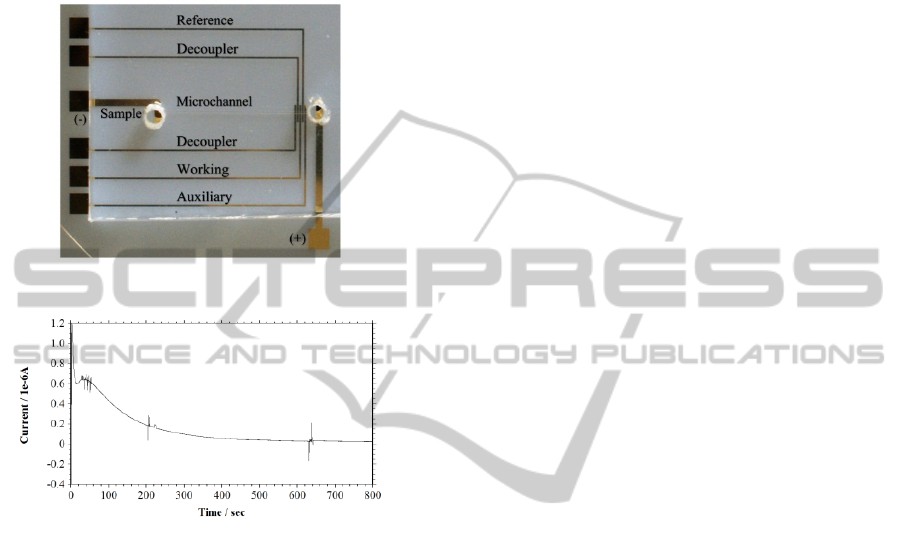
the i-t curve of MB-DNA detection through CE-AD.
Different peaks resemble MB attached to various
bands of the DNA. The peak was detected after 2
min of sample injection. The detection sensitivity
and LOD for this reaction were 640 nA/μg of DNA
and 140 ng of DNA respectively.
Figure 5: CE-AD microchip.
Figure 6: i-t curve of MB-DNA complex in KCl 200 mM.
Therefore, it would be possible to first determine
peak current for MB from CE-AD experiment and
then decipher its concentration using standard curve
obtained under control conditions (Figure 4).
Thereafter, DNA concentration can be calculated
from the standard value obtained. This shows the
potential of present study towards successful
detection of DNA of any sizes and deciphers its
concentration on our microchip. Further studies are
underway to enhance the reproducibility and
sensitivity of these devices and for detection of a
mixture of short DNA fragments such as found in
DNA molecular weight markers.
4 CONCLUSIONS
It was concluded in the present study that disposable
CE-AD microchips with gold electrodes and PDMS
channels can be used to prepare effective DNA
detector in place of existing gel electrophoresis
based system. The disposable electrochemical
detector fabricated in this study displayed good
performance in terms of sensitivity, stability,
resolution and peak density. This type of microchip
can also be used for detection of various other
organic or inorganic compounds, or can be
integrated with microfluidic modules on a µTAS.
REFERENCES
In-Je Yi, Ju-Ho Kim, Y. J. Choi, C. J. Kang, Yong-Sang
Kim, 2006. A disposable biosensor with Prussian blue
deposited electrode, Microelectronic Engineering, 83
(2006) 1594–1597.
Ju-Ho Kim, C. J. Kang, Yong-Sang Kim, 2005.
Development of a microfabricated disposable
microchip with a capillary electrophoresis and
integrated three-electrode electrochemical detection,
Biosensors and Bioelectronics 20 (2005) 2314–2317.
Gi-Sung Joo, Sandeep Kumar Jha, Yong-Sang Kim, 2009.
A capillary electrophoresis microchip for
amperometric detection of DNA, Current Applied
Physics 9 (2009) e222–e224.
Dolnik V., Liu S., Jovanovich S., 2000. Capillary
electrophoresis on microchip, Electrophoresis, 27, 41–
54.
Woolley A. T., Lao K., Glazer A. N., Mathies R. A., 1998.
Capillary Electrophoresis Chips with Integrated
Electrochemical Detection, Analytical Chemistry, 70,
684–688.
Jacobson S. C., Koutny L. B., Hergenroder R., Moore A.
W., Ramsey J. M., 1994. Effects of Injection Schemes
and Column Geometry on the Performance of
Microchip Electrophoresis Devices, Analytical
Chemistry, 66, 1107–1113.
Culbertson C. T., Jacobson S. C., Ramsey J. M., 2000.
Microchip Devices for High-Efficiency Separations,
Analytical Chemistry, 72, 5814–5819.
MICROCHIP CAPILLARY ELECTROPHORESIS DEVICE FOR AMPEROMETRIC DETECTION OF DNA WITH
REDOX INTERCALATION
287
