
THE CONSEQUENCES OF LOW FREQUENCY AND INTENSITY
ELECTROMAGNETIC FIELDS ON THE FREQUENCY OF
MICRONUCLEI IN HeLa CELLS
Cosmin Teodor Mihai
1
, Gabriela Căpraru
2
, Elena Truţă
2
, Pincu Rotinberg
2
and Daniela Gherghel
2
1
Faculty of Biology,“Al. I. Cuza” University, Bdul Carol I nr. 20A, Iasi, Romania
2
Biological Research Institute, Str. Lascar Catargi nr. 47, Iasi, Romania
Keywords: Low frequency electromagnetic fields, HeLa neoplastic cells, Micronuclei assay, Genetic effects.
Abstract: The treatment of HeLa neoplastic cells with low frequency and intensity electromagnetic field has
determined modifications of the micronuclei number, this impact being correlated with the application
manner of the electromagnetic field (continuous or discontinuous). Thus, the continuous electromagnetic
field has reduced the frequency of the micronuclei formation (2.91 ± 0.015 ‰), as compared to the value of
control group (3.93 ± 0.023 ‰), while the discontinuously applied electromagnetic field has increased the
number of micronuclei (4.92± 0.012 ‰). These variations in micronuclei number suggested that low
frequency electromagnetic field interfere in different ways with the genetic material of cancerous cells,
indicating that the cEMF had a protective effect upon DNA molecule, while dcEMF had a genotoxic impact.
Also, the estimation of the micronuclei area has revealed that the area of micronuclei generated by dcEMF
was smaller than that of cEMF.
1 INTRODUCTION
Low frequency and intensity electromagnetic fields
are a part of our life due to the large scale utilization
of computers, home appliances, radio
communications or of the other electrical devices.
Now, everyone is living in a mix of weak electric
and magnetic fields, the impact upon our organism
being still under investigation. The majority of
studies have investigated the possible negative
effects of low frequency and intensity
electromagnetic fields upon humans (Hardell &
Sage, 2008; Heynick, Johnston & Mason, 2003;
Johansson, 2009; Kavet, 1996; Verschaeve et al.,
2006).
A few studies have been oriented towards the
investigation of the impact of the electromagnetic
fields upon the cancerous cells and the evaluation of
consequences of the exposure on this type of cells to
the low frequency and intensity EMF (Falone et al.,
2007; Girgert, Gründker, Emons & Hanf, 2008;
Ronchetto et al., 2004; Tenuzzo et al., 2006). The
findings obtained on this experimental model are
contradictory and full of gaps due to the lack of an
unitary exposure setup, protocol or used biological
material.
The necessity to understand and investigate the
possible functional interactions between
electromagnetic fields and cancerous cells is
determined by the fact that electromagnetic fields
are incriminated to facilitate the carcinogenicity
(Juutilainen & Lang, 1997; Juutilainen, Kumlin &
Naarala, 2006; Mairs et al., 2007; Meltz, 2003;
Simkó, Kriehuber, Weiss & Luben, 1998; Thun-
Battersby, Mevissen & Löscher, 1999). From this
point of view is important to identify the most
dangerous characteristics (frequency, intensity,
amplitude modulations, time of exposure) of
electromagnetic fields to limit the influence upon
healthy or tumor bearing persons. One of the ways
to estimate the impact of electromagnetic fields upon
cells is represented by quantification of micronuclei
occurrence in the exposed cells.
The micronuclei (MN) test is the most frequent
technique used to detect chromosome breakage or
mitotic interference of different xenobiotics, events
thought to be associated with increased risk for
cancer or with a supplementary genetic
destabilization of already mutated cells (like
cancerous ones) (Wolff & Muller, 2005).
440
Mihai C., C
ˇ
apraru G., Tru¸t
ˇ
a E., Rotinberg P. and Gherghel D..
THE CONSEQUENCES OF LOW FREQUENCY AND INTENSITY ELECTROMAGNETIC FIELDS ON THE FREQUENCY OF MICRONUCLEI IN HeLa
CELLS.
DOI: 10.5220/0003292804400443
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 440-443
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
This work presents an initial investigation about
the impact of the low frequency and intensity
electromagnetic field upon the integrity of HeLa's
genetic material by registration of the variations in
the frequency of the micronuclei occurrence.
2 MATERIAL AND METODS
The biological material used in the in vitro
experiments was represented by mycoplasm-
negative HeLa cellular cultures of human neoplastic
origin.
HeLa cells were cultured in DMEM medium
(Dulbeco's Modified Eagle’s Medium, Biochrom
AG, Germany, FG 0415), supplemented with 10.0%
fetal bovine serum (Sigma, Germany, F9665), 100
µg/mL streptomycin (Biochrom AG, Germany,A
331-26), 100 IU/mL penicillin (Biochrom AG,
Germany, A 321-44) and 50 µg/mL antimycotic
amphotericin B (Biochrom AG, Germany, A 2612),
at a density of 5 x 10
5
cells / 25 cm
2
flask, at 37
o
C
When the cells reached confluence in the monolayer
stage, the cultures were divided into control and
electromagnetic treated cell cultures.
The electromagnetic field (EMF) of continuous
or discontinuous type (cEMF, dcEMF) was
generated by an IBF magnetodiaflux device. This
presents two circular coils (29 cm in diameter,
placed at a distance of 14.5 cm) disposed on a
cardboard cylinder, which delimits the place where
the culture flasks are maintained during the
electromagnetic treatment. The intensity and
frequency of the generated electromagnetic field
were of 5.5 mT and 100 Hz.
Single EMF was applied continuously or
discontinuously (with breaks of 1 second and action
3 seconds) to the cell cultures, for a period up to 60
minutes. Simultaneous experiments skipping the
electromagnetic field were also performed on the
control cultures. During the real or blind treatment
the cell cultures were removed from the incubator
and placed into magnetodiaflux, where the
temperature has reached up to 30
0
C.
After the electromagnetic treatment, the cell
cultures were left for another 24 hours in incubator
and then were subjected to quantification of the
micronuclei number. First, the medium was
discarded, the cell layer was rinsed with PBS
(phosphate buffer saline) and than was detached
from the surface of the flasks with a solution of
trypsin (Biochrom AG, Germany).
The cell suspension was resuspended in normal
growth medium and centrifuged at 1800 rpm for 2
minutes. Over the pellet was added fresh and cooled
Carnoy's fixative. The slides preparation was made
by air-dry method and then observed at a Nikon
Eclipse 600 microscope, at the magnification of
4000x.
The frequency of micronuclei was calculated as
the number of micronuclei at 1000 read interphases.
The determination of the micronuclei area was
realized with ImageJ software and the calculation of
the approximate surface of micronuclei was based
on the circle area formula.
The results are expressed as mean ± SE and were
statistically analyzed by Students‘t’ test. The p value
<0.05 was considered significant.
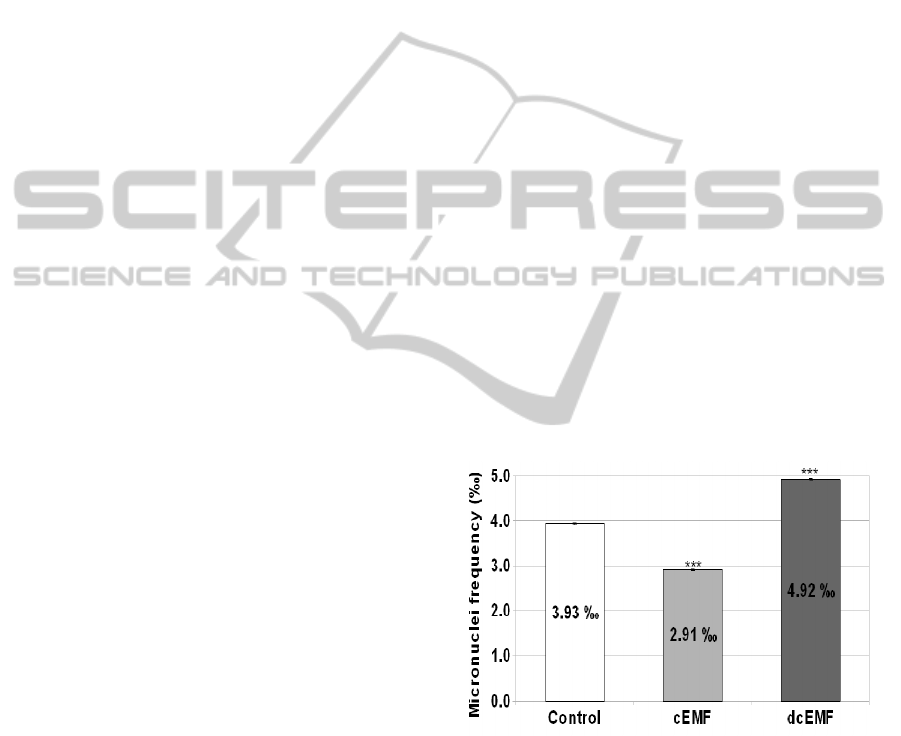
3 RESULTS AND DISCUSSIONS
The evaluation of the consequences determined by
the exposure of HeLa cells to the continuously or
discontinuously applied electromagnetic fields upon
the genetic material integrity was based on the
estimation of the micronuclei occurrence frequency
to 1000 interphases, as compared to the specific MN
frequency of the control group (Figure 1). The
micronuclei occurrence frequency in the control
group was of 3.93 ± 0.023 ‰, value which was
considered by us as reference and was equated to
100%.
Figure 1: Micronuclei occurrence frequency (expressed as
procentual values) in the case of the HeLa neoplastic cell
cultures untreated or treated with continuous or
discontinuous electromagnetic field (100 Hz, 5.5 mT) for
60 minutes. Significantly different from control: *p<0.05,
**p<0.01, ***p<0.001.
As compared to the control group, the
electromagnetic treatment applied continuously has
determined a reduction of the micronuclei frequency
to the value of 2.91 ± 0.015 ‰, corresponding to a
procentual depletion of 25.84%.
THE CONSEQUENCES OF LOW FREQUENCY AND INTENSITY ELECTROMAGNETIC FIELDS ON THE
FREQUENCY OF MICRONUCLEI IN HeLa CELLS
441

Contrary, the discontinuous electromagnetic field
has induced an augmentation of the micronuclei
frequency, the registered value (4.92± 0.012 ‰)
being with 25.20% over the reference.
Another approach of the research was the
estimation of the micronuclei total area, determined
using photos, as an attempt to quantify the amount
of genetic material expelled with micronuclei (Table
1).
Table 1: Mean micronuclei area (μm
2
) and procentual
variations specific to the control group and to the treated
groups either with cEMF or with dcEMF.
Experimental
group
Micronuclei total area
( μm
2
)
% variation
Control 13.01 100
cEMF 50.10 385.09
dcEMF 20.59 158.26
The mean area of the micronuclei of the control
group was of 13.01 μm
2
, while the groups treated
with cEMF and dcEMF, respectively, have presented
a mean area of 50.10 μm
2
and 20.59 μm
2,
respectively.
Ionizing radiations along with other numerous
chemical mutagens causes structural chromosomal
aberrations, some of them being visible at the light-
microscope level. The aberrations from the level of
chromosome can generate chromosome fragments
without spindle attachment organelles, being called
acentric fragments. When the cell divides, some of
these fragments are excluded from the main
daughter nuclei and form small extra nuclei within
the cytoplasm, either on their own, or in conjunction
with other fragments. Such "micronuclei" (MN) can
appear in the cytoplasm of either, or both, daughter
cells (Savage, 2000)
The electromagnetic field treatment,
continuously or discontinuously applied, has
determined, as compared to the reference value,
fluctuations in the micronuclei occurrence
frequency. These effects, specific for every type of
electromagnetic field, are due to probably different
patterns of interaction between EMFs and the
genetic material of the HeLa cells.
Our presumption regarding the reduced
frequency of micronuclei in the experimental group
treated with cEMF is that cEMF either modifies the
electric charge of DNA molecule, followed by a
stabilization of the genetic material, or intensifies
the activity of molecular mechanisms responsible for
maintaining the DNA integrity. Also, changes in the
electrical charge of DNA macromolecule could
explain the higher area of micronuclei, as compared
to control group and dcEMF. In this case, the
breakage of the DNA structure could occur in the
zones where the fragility of DNA is higher,
generating fragments of genetic material.
The elevated number of micronuclei in cells
treated with dcEMF suggests the weakening of the
DNA integrity either by induction by dcEMF of
microoscilations in the structure of DNA, with the
generation of suplimentary breakages, or by
modification of the intracellular microenvironoment
constants, with brutal and destructive consequences
upon genetic material integrity (Davies et al., 1999).
As in the case of cEMF, the area of micronuclei after
dcEMF action was higher than that of the reference
group, but smaller than of cEMF, suggesting the
release of shorter DNA fragments.
From the above presented data, we can conclude
the existence of an inverse relationship between
frequency of micronuclei and their area, suggesting
different sites of interaction of EMF with DNA, with
immediate consequences upon its integrity.
Our experimental data, obtained in this
experimental frame, are overlapping with those from
the specialty literature (that contains limited
references to cancerous cells) - which has signaled
the sporadic existence (Hee Cho & Chung Won,
2003; Juutilainen, Heikkinen, Soikkeli & Mäki-
Paakkanen, 2007) or even absence (Pasquini et al.,
2003; Speit, Schütz & Hoffmann, 2007; Verschaeve
et al., 2006) of some genotoxic effects induced by
electromagnetic fields, generator of micronuclei.
4 CONCLUSIONS
The EMF interaction with neoplastic HeLa cells has
generated an increase (dcEMF) or a decrease
(cEMF) of the micronuclei occurrence frequency,
suggesting different sites and ways of action upon
the genetic material of cancerous cells.
cEMF expressed a protector effect upon genetic
integrity of HeLa cells, while the dcEMF had a
genotoxic impact upon DNA molecule.
Software analysis of the expelled micronuclei
area has showed that EMF has generated
micronuclei with higher areas than those in the case
of the control group and allowed the establishment
of an inverse relationship between micronuclei
occurrence frequency and their areas.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
442

ACKNOWLEDGEMENTS
This study was possible with financial support from
the Sectoral Operational Programme for Human
Resources Development, project “Developing the
innovation capacity and improving the impact of
research through post-doctoral programmes”,
POSDRU/89/1.5/S/49944
REFERENCES
Davies, E., Olliff, C., Wright, I., Woodward, A. & Kell,
D. (1999). A weak pulsed magnetic field affects
adenine nucleotide oscillations, and related parameters
in aggregating Dictyostelium discoideum amoebae.
Bioelectrochem Bioenerg, 48, 149-162.
Falone, S., Grossi, M. R., Cinque, B., D'Angelo,
B., Tettamanti, E., Cimini, A., Di Ilio, C. & Amicarelli,
F. (2007). Fifty hertz extremely low-frequency
electromagnetic field causes changes in redox and
differentiative status in neuroblastoma cells. Int. J.
Biochem. Cell Biol., 39, 2093-2106.
Girgert, R., Gründker, C., Emons, G. & Hanf, V. (2008).
Electromagnetic fields alter the expression of estrogen
receptor cofactors in breast cancer cells.
Bioelectromagnetics, 29, 169-176.
Hardell, L. & Sage, C. (2008). Biological effects from
electromagnetic field exposure and public exposure
standards. Biomed. Pharmacother., 62, 104-109.
Hee Cho, Y. & Chung Won, H. (2003). The effect of
extremely low frequency electromagnetic fields (ELF-
EMF) on the frequency of micronuclei and sister
chromatid exchange in human lymphocytes induced
by benzo(a)pyrene. Toxicol. Lett., 143, 37-44.
Heynick, L. N., Johnston, S. A. & Mason, P.A. (2003).
Radio frequency electromagnetic fields: cancer,
mutagenesis, and genotoxicity. Bioelectromagnetics,
Suppl 6, S74-100.
Johansson, O. (2009). Disturbance of the immune system
by electromagnetic fields-A potentially underlying
cause for cellular damage and tissue repair reduction
which could lead to disease and impairment.
Pathophysiology, 16, 157-177.
Juutilainen, J. & Lang, S. (1997). Genotoxic, carcinogenic
and teratogenic effects of electromagnetic fields.
Introduction and overview. Mutat. Res., 387, 165-171.
Juutilainen, J., Heikkinen, P., Soikkeli, H. & Mäki-
Paakkanen, J. (2007).Micronucleus frequency in
erythrocytes of mice after long-term exposure to
radiofrequency radiation. Int. J. Radiat. Biol., 83, 213-
220.
Juutilainen, J., Kumlin, T. & Naarala, J. (2006). Do
extremely low frequency magnetic fields enhance the
effects of environmental carcinogens? A meta-analysis
of experimental studies. Int. J. Radiat. Biol., 82, 1-12.
Kavet, R. (1996). EMF and current cancer concepts.
Bioelectromagnetics, 17, 339-357.
Mairs, R. J., Hughes, K., Fitzsimmons, S., Prise, K. M.,
Livingstone, A., Wilson, L., Baig, N., Clark, A. M.,
Timpson, A., Patel, G., Folkard, M., Angerson, W. J. &
Boyd, M. (2007). Microsatellite analysis for
determination of the mutagenicity of extremely low-
frequency electromagnetic fields and ionising
radiation in vitro. Mutat. Res., 626, 34-41.
Meltz, M. L. (2003). Radiofrequency exposure and
mammalian cell toxicity, genotoxicity, and
transformation. Bioelectromagnetics, Suppl 6, S196-
213.
Pasquini, R., Villarini, M., Sforzolini Scassellati, G.,
Fatigoni, C. & Moretti, M. (2003). Micronucleus
induction in cells co-exposed in vitro to 50 Hz
magnetic field and benzene, 1,4-benzenediol
(hydroquinone) or 1,2,4-benzenetriol. Toxicology in
Vitro, 17, 581-586.
Ronchetto, F., Barone, D., Cintorino, M., Berardelli, M.,
Lissolo, S., Orlassino, R., Ossola, P. & Tofani, S.
(2004). Extremely low frequency-modulated static
magnetic fields to treat cancer: A pilot study on
patients with advanced neoplasm to assess safety and
acute toxicity. Bioelectromagnetics, 25, 563-571.
Savage, J. R. K. (2000). Micronuclei : Pitfalls and
Problems in Atlas of Genetics and Cytogenetics in
Oncology and Haematology. Retrived July 2000 from
http://atlasgeneticsoncology.org/Deep/MicronucleiID2
0016.html.
Simkó, M., Kriehuber, R., Weiss, D. G. & Luben, R.A.
(1998). Effects of 50 Hz EMF exposure on
micronucleus formation and apoptosis in transformed
and nontransformed human cell lines.
Bioelectromagnetics, 19, 85-91.
Speit, G., Schütz, P. & Hoffmann, H. (2007). Genotoxic
effects of exposure to radiofrequency electromagnetic
fields (RF-EMF) in cultured mammalian cells are not
independently reproducible. Mutat. Res., 626, 42-47.
Tenuzzo, B., Chionna, A., Panzarini, E., Lanubile, R.,
Tarantino, P., Di Jeso, B., Dwikat, M. & Dini, L.
(2006). Biological effects of 6 mT statistic magnetic
fields: a comparative study in different cell types.
Bioelectromagnetics, 27, 560-577.
Thun-Battersby, S., Mevissen, M. & Löscher, W. (1999).
Exposure of Sprague-Dawley rats to a 50-Hertz, 100-
microTesla magnetic field for 27 weeks facilitates
mammary tumorigenesis in the 7,12-dimethylbenz[a]-
anthracene model of breast cancer. Cancer Res., 59,
3627-3633.
Verschaeve, L., Heikkinen, P., Verheyen, G., Van Gorp, U.,
Boonen, F., Vander Plaetse, F., Maes, A., Kumlin, T.,
Mäki-Paakkanen, J., Puranen, L. & Juutilainen, J.
(2006). Investigation of co-genotoxic effects of
radiofrequency electromagnetic fields in vivo. Radiat.
Res., 165, 598-607.
Wolff, I. & Muller, P. (2005). Micronuclei and Comet
Assay. In J. E. Celis, T. Hunter, N. Carter, D. Shotton,
J. V. Small & K. Simons (Eds.), Cell Biology: A
Laboratory Handbook. Elsevier Academic Press. pp.
325-334.
THE CONSEQUENCES OF LOW FREQUENCY AND INTENSITY ELECTROMAGNETIC FIELDS ON THE
FREQUENCY OF MICRONUCLEI IN HeLa CELLS
443
