
MULTI-ANALYTE DETECTION FOR BIOLOGICAL FLUIDS
Towards Continous Monitoring of Glucose, Ionized Calcium and pH
using a Viscometric Affinity Biosensor
Christophe Boss, Eric Meurville, Peter Ryser
Laboratory of Microengineering for Manufacturing, EPFL, Lausanne, Switzerland
Frédéric Schmitt, Lucienne Juillerat-Jeanneret
Pathology Institute, University Hospital, Lausanne, Switzerland
Pablo Dosil-Rosende, Desdemona De Souza
Baxter Healthcare, Zurich, Switzerland
Keywords: Affinity sensor, Chemico-mechanical sensor, Glucose sensing, Concanavalin A, Dextran, Viscosity.
Abstract: We present a viscometric affinity biosensor that can potentially allow continuous multi-analyte monitoring
in biological fluids like blood or plasma. The sensing principle is based on the detection of viscosity
changes of a polymeric solution which has a selective affinity for the analyte of interest. The chemico-
mechanical sensor incorporates an actuating piezoelectric diaphragm, a sensing piezoelectric diaphragm and
a flow-resisting microchannel for viscosity detection. A free-standing Anodic Alumina Oxide (AAO)
porous nano-membrane is used as selective interface. A glucose-sensitive sensor was fabricated and
extensively assessed in buffer solution. The sensor reversibility, stability and sensitivity were excellent
during at least 65 hours. Results showed also a good degree of stability for a long term measurement (25
days). The sensor behaviour was furthermore tested in fetal bovine serum (FBS). The obtained results for
glucose sensing are very promising, indicating that the developed sensor is a candidate for continuous
monitoring in biological fluids. Sensitive solutions for ionized calcium and pH are currently under
development and should allow multi-analyte sensing in the near future.
1 INTRODUCTION
Continuous detection overtime of analyte levels in
complex biological fluids such as blood or plasma is
a tricky task since strong interferences from other
biomolecules may occur during the measurements.
A successful system for continuous monitoring of
physiologically relevant parameters would afford
great benefits in numerous pathologies such as in the
thrombosis with the assessment of blood calcium
levels, in diabetes with glucose concentrations or pH
in acidosis/alkalosis disorders. In addition, these
parameters are also of primary importance for
critically ill patients. Today, these controls are
performed by hand and continuous monitoring could
contribute to reduce the risk of mortality in the
intensive care units.
Existing detection methods are currently based
on electrochemical principles, which have
limitations for in vivo monitoring. Electrochemical
measurements depend on the analyte diffusion rate.
Consequently, biofouling affects the sensitivity and
frequent calibrations are required. Furthermore, the
presence of other electrochemically active solutes
often produces inaccuracies. An alternative approach
which could overcome these limitations is affinity
sensing. Affinity sensing is more tolerant to
biofouling, which results only in an increased
stabilization time, and is intrinsically not subjected
to electroactive interferences. For these reasons,
intensive investigations on affinity binding sensors
have been carried out using different technics such
as fluorescence (Ballerstadt, 2004) or viscosity
measurements (Huang, 2009). Recently, hydrogel-
based sensors have emerged as promising materials
295
Boss C., Meurville E., Ryser P., Schmitt F., Juillerat-Jeanneret L., Dosil-Rosende P. and De Souza D..
MULTI-ANALYTE DETECTION FOR BIOLOGICAL FLUIDS - Towards Continous Monitoring of Glucose, Ionized Calcium and pH using a Viscometric
Affinity Biosensor.
DOI: 10.5220/0003350902950298
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 295-298
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
for affinity sensing (Tierney, 2009). Despite many
efforts towards the development of continuous
biosensors for medical and biological applications,
long term reversibility and stability remains a
challenge.
In this context, we propose a novel chemico-
mechanical method which aims at detecting
viscosity changes of a solution which has a selective
affinity for the analyte of interest (Fig. 1). A semi-
permeable membrane ensures that the analyte
concentration in the biosensor is the same as in the
patient blood or plasma. The viscosity detection of
the sensitive solution is based on a microchannel
which exhibits a resistance to the flow circulating
through it. The sinusoidal actuation of a
piezoelectric diaphragm generates a flow through
the microchannel which results in a deflection of the
sensing piezoelectric diaphragm, inducing a voltage
which can be recorded. The phase shift between the
applied voltage and the sensing piezoelectric
diaphragm deflection is a measurement of the fluid
viscosity.
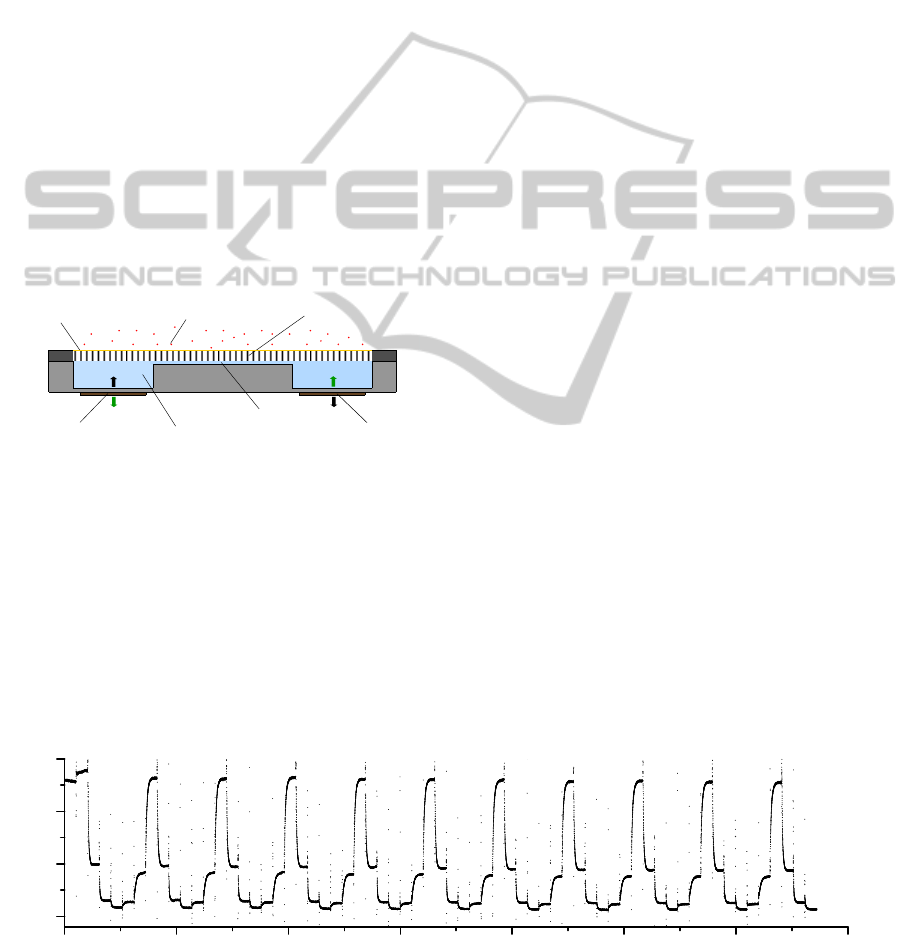
Analyte
Actuating
diaphragm
Sensing diaphrag
m
Free-standing AAO
porous nano-membrane
Sensitive solution
Microchannel
Anti-biofouling coating
Figure 1: Schematic illustration of the biosensor principle.
(Cross view).
A proof of concept of continuous glucose
monitoring was previously reported using a
macroscopic demonstrator (Boss, 2009). The present
paper investigates the sensitivity, reversibility and
reproducibility in a buffer solution of a novel
glucose sensor fabricated by stereolithography. The
sensor behavior in biological fluids was also
assessed using fetal bovine serum (FBS). The
glucose-sensitive fluid used inside the sensor was
based on Concanavalin A (ConA), a protein which
specifically binds to glucose, and a high-molecular-
weight dextran. When glucose concentration
increases, dextran is partially replaced by glucose at
the binding sites of ConA. As a result, the network
ConA-dextran is weakened, and the viscosity of the
sensing fluid decreases.
2 MATERIALS AND METHODS
2.1 Sensor Fabrication
The sensor was fabricated by stereolithography
using a biocompatible resin specially dedicated to
medical applications (Proform). The actuating
diaphragm was made up of a 50 μm thick and 3 mm
in diameter lead zirconate titanate (PZT) disc
(Audiowell Electronics) glued on a 10 μm thick
brass foil (Goodfellow). The sensing diaphragm was
made up of a 28 μm thick and 3 mm in diameter
Polyvinylidene fluoride (PVDF) disc (Measurement
Specialties) glued on a 10 μm thick brass foil. The
semi-permeable membrane was a 50 μm thick
Anodic Aluminium Oxide (AAO) membrane with
4-6 nm in diameter pores (Synkera Technologies). A
100×100 μm
2
in section glass capillary was used as
microchannel. The sensor assembly was realized
using medical adhesive epoxy (Loctite M-21HP).
The sensor was 200 μm thick and the volume of
sensitive fluid encapsulated inside was 4 μL. The
sensitive fluid was prepared using the protocol
described by Kuenzi et al. (2000). The sensitive
fluid was composed of 2% [w/w] dextran 3200
(PSS) and 0.4% [w/w] ConA (Sigma). The viscosity
of the sensitive fluid ranged from 5.9-16.7 mPas
(30-2 mM glucose) at 25°C and from 4.2-9.4 mPas
(30-2 mM) at 37°C. A low viscosity was selected to
keep the glucose diffusion as fast as possible.
0 10203040506070
20
30
40
50
Phase shift [deg]
Time [hour]
Figure 2: Phase shift response to multiple glucose concentrations (2, 6, 12, 20 mM). Measurement performed in reference
solution at 25°C.
20 mM
12 mM
6 mM
2 mM
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
296

0 5 10 15 20 25
28
30
32
34
36
38
Phase shift [deg]
Time [day]
Figure 3: Long term glucose concentration measurement in reference solution at 37°C, between two physiologically
relevant glucose concentrations, 2 mM and 12 mM.
2.2 Experimental Setup
Glucose measurements were performed in a
reference solution which is an isotonic solution of
the sensitive fluid, but without dextran and ConA.
Stock solutions with different glucose concentrations
were subsequently pumped into the test cell using a
computer controlled syringe pump. The whole setup
was located in a thermally regulated chamber
(±0.01°C), as the viscosity of the sensitive fluid is
strongly temperature dependant.
Fetal bovine serum (FBS) was preserved frozen.
Before use, 0.1% of sodium azide (NaN
3
) was added
as preservative. FBS was heated at 56°C during
45 min to inactivate the complement system. FBS
was then filtered (Millex HV 0.45 μm syringe filter)
to remove aggregates which may clog the semi-
permeable membrane. The glucose concentration of
FBS was measured using a standard glucose meter
(Accu-Chek). Concentrated (2M) D-glucose solution
was added to increase the FBS glucose
concentration.
3 RESULTS AND DISCUSSION
3.1 Reversibility of Glucose-induced
Viscosity Change
In Figure 2, a sensor is exposed successively to
increasing and decreasing glucose concentrations (2,
6, 12, 20 mM). Ten full cycles were performed
during 65 hours showing an excellent reversibility
and stability. The response time of the sensor (time
to reach 90% of the final value) was 4.8 min for
increasing glucose concentration and 19.3 min for
decreasing glucose concentration. The longer time
constant for decreasing glucose concentration is
explained by the smaller mobility of glucose
molecules in the viscous sensitive fluid than in the
reference solution. The semi-permeable membrane
porosity affects therefore more glucose molecules
diffusing out of the sensor than glucose molecules
entering inside the sensor. At this stage,
miniaturization was not the primary focus and the
response time is over the 10 min required from a
medical point of view. The response time could be
shorted by reducing the sensor thickness or sensitive
fluid viscosity. The sensor sensitivity in the
physiological and hypoglycemic ranges of glucose
concentration (2-6 mM) was 0.1 mM, which is
accurate enough for patients monitoring.
3.2 Long Term Stability
The long term stability of the sensor at 37°C was
investigated (Fig. 3). Glucose concentrations were
changed every 12 hours during 25 days. The sensor
showed a remarkable stability over time, but a
progressive loss of sensitivity was nevertheless
observed. After 25 days, the sensor sensitivity
dropped to 73% of the initial sensitivity. The loss of
sensitivity was likely due to ConA leakage through
the biggest pores and defects of the 4-6 nm pores of
the semi-permeable membrane. We are currently
assessing new AAO porous nano-membranes with
reduced pores size (2-4 nm) which should improve
the long term stability of the sensor.
3.3 Determination of Glucose in Fetal
Bovine Serum
The sensor behaviour in complex biological fluids
was evaluated using fetal bovine serum (FBS). Fig.
4 shows the sensor response to glucose variation (2.6
and 30 mM) in FBS at 37°C. Twelve full cycles
were performed during 24 hours, showing a good
stability. The response time did not increase with
time indicating that biofouling due to protein
adsorption on the semi-permeable membrane is not
an issue. AAO porous nano-membranes are
therefore well-suited as selective interface for
biosensors intended to be used in biological fluids.
2 mM
12 mM
MULTI-ANALYTE DETECTION FOR BIOLOGICAL FLUIDS - Towards Continous Monitoring of Glucose, Ionized
Calcium and pH using a Viscometric Affinity Biosensor
297

30.0
30.2
30.4
30.6
30.8
31.0
31.2
0 5 10 15 20 25
Time [hour]
Phase shift [deg]
Figure 4: Measurement in fetal bovine serum at 37°C between 2.6 mM and 20 mM.
The sensor sensitivity in FBS dropped to 10% of the
sensitivity in buffer solution. After FBS testing, the
sensor recovered its initial sensitivity in buffer
solution. The loss of sensitivity is therefore
reversible, which leads to the following hypothesis.
Small glycosylated peptides may enter inside the
sensor and competitively interfere with the binding
reaction of ConA to glucose. This hypothesis was
confirmed by conducting similar experiments with
dialysed FBS. A loss of sensitivity of 30% and 40%
were observed in 12 kDa and 3.5 kDa dialysed FBS,
respectively. The pores size should therefore be
significantly reduced to prevent glycosylated
peptides from entering the sensor. We are currently
assessing 2-4 nm AAO nano-membranes, coated
with Al
2
O
3
by atomic layer deposition, to minimize
the pores size.
4 CONCLUSIONS
A viscosity-based affinity sensor was developed for
continuous monitoring in biological fluids. The
sensor was extensively tested in buffer solution,
showing an excellent reproducibility and stability
over 65 hours at 25°C. The sensor sensitivity
matched well within the hypoglycemic and
physiological ranges (2-6 mM) with a resolution of
0.1 mM. The response time of the sensor was higher
for decreasing glucose concentration due to the
conjugated effect of both the reduced mobility of
glucose molecules in the viscous sensitive solution
and the membrane porosity. The sensor showed also
remarkable long term stability (25 days) at 37°C. A
limited loss of sensitivity was nevertheless observed,
which may be explained by ConA leakage through
defects of the AAO porous nano-membrane. In FBS,
the response time did not increase with time,
indicating that biofouling due to protein adsorption
is not an issue. The sensitivity in non-dialysed and
12 kDa dialysed FBS were 10% and 60% of the
sensitivity in buffer solution, respectively.
Glycosylated perptides may enter inside the sensor
and interfere with the ConA-glucose reaction. The
loss of sensitivity in FBS should be solved by
reducing the membrane pores size.
These measurements show good promise for the
sensor to be applied as in vivo monitoring system.
We are currently assessing Al
2
O
3
coated 2-4 nm
AAO nano-membranes for pores size reduction,
which should allow sensitive measurements in FBS.
Sensitive fluids for ionized calcium and pH are also
under development and should allow multi-analyte
sensing in the near future.
REFERENCES
Ballerstadt, R., Polak, A., Beuhler, A., Frye, J., 2004. In
Vitro long-term performance study of a near-infrared
fluorescence affinity sensor for glucose monitoring.
Biosensors & Bioelectronics. Elsevier.
Boss, C., Meurville, E., Sallese, J.M., Ryser, P., 2009.
Novel chemico-mechanical approach towards long-
term implantable glucose sensing. Procedia
Chemistry. Elsevier.
Huang, X., Li, S., Schultz, J., Wang, Q., Lin, Q., 2009. A
MEMS affinity glucose sensor using a biocompatible
glucose-responsive polymer. Sensor and Actuators B:
Chemical. Elsevier.
Huang, X., Li, S., Schultz, J., Wang, Q., Lin, Q., 2009. A
Capacitive MEMS Viscometric Sensor for Affinity
Detection of Glucose. J Micromech Microeng. IEEE.
Kuenzi, S., Meurville, E., Ryser, P., 2010. Automated
characterization of dextran/concanavalin A mixtures –
A study of sensitivity and temperature dependence at
low viscosity as basis for an implantable glucose
sensor. Sensor and Actuators B: Chemical. Elsevier.
Tierney, S., Falch, B., Hjelme, D., Stokke, B., 2009.
Determination of Glucose Levels Using a
Functionalized Hydrogel-Optical Fiber Biosensor:
Toward Continuous Monitoring of Blood Glucose in
Vivo. Anal. Chem. ACS.
20 mM
2.6 mM
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
298
