
LEARNING FROM BIOFEEDBACK
Patient-specific Games for Neuromuscular Rehabilitation
Ouriel Barzilay and Alon Wolf
Biorobotics and biomechanics Laboratory, Technion - Israel Institute of Technology, Haifa, Israel
Keywords: Artificial neural networks, Patient-specific rehabilitation, Virtual reality, Biofeedback.
Abstract: Rehabilitation tasks are generally subjected to the physiotherapist’s qualitative interpretation of the patient’s
pathology and needs. Motivated by the recently increasing use of virtual reality in rehabilitation, we propose
a novel approach for the design of those biomechanical tasks for an improved patient-specific and
entertaining rehabilitation. During training, the subject wears 3D goggles in which virtual tasks are
displayed to him. His kinematics and muscles activation are tracked in real time and an inverse model is
estimated by artificial neural networks. The resulting inverse model produces a physical exercise according
to the observed abilities of the subject and to the expected performance dictated by the physiotherapist. The
system offers several advantages to both the patient and the physiotherapist: the tasks can be presented in
the form of interactive personalized 3D games with augmented feedback, stimulating the patient’s
motivation and reducing the need of constant monitoring from the therapist. Additionally, offline
quantitative data from every training session can be stored for further analysis. The results of our study on
arm movements suggest an improvement in the training efficiency by 10% for the biceps and by 32%
(p=0.02) for the triceps.
1 INTRODUCTION
Physiotherapy aims at helping patients recover
maximal movement and functionality after surgical
operations, injuries or strokes. Neuromuscular
rehabilitation is generally performed in the form of
biomechanical tasks, designed to restore a cognitive
or mechanical function within the patient. These
tasks are elaborated by the physiotherapist, based on
his diagnosis of the patient’s pathology. Success of
the rehabilitation training relies on the adequate
design of these tasks, on the repetition of the
physical exercises by the patient, on the subject’s
motivation and on the feedback to the patient
(Holden, 2005). Furthermore, for good results, the
task must be adapted to the actual performance of
the patient. This adaptive physiotherapy is very
difficult to perform online and is subjected to the
trainer’s interpretation of the patient's performance.
Today, rehabilitation in virtual reality has
become a large field of research and several studies
have been published on the efficiency and the
advantages offered by this approach. Virtual
rehabilitation consists in the execution of
biomechanical tasks in virtual environments,
generally by the means of display devices,
biofeedback or haptic instrumentation and adapted
software. Virtual rehabilitation has proved efficient
in the treatment of neurological diseases (e.g. in Jack
et al., 2001 or Holden et al., 2005) for patients with
balance disorders (Jacobson et al., 2001) or sports
medicine. Studies have shown scientific evidence
that motor skills can be learned in virtual
environments (Regian et al., 1992) and transferred to
the real world (Holden and Dyar, 2002).
Furthermore, the augmented feedback on
performance offered by virtual reality improves the
results of rehabilitation (Shea and Wulf, 1999). It is
also likely to increase the motivation of the patient
during training (Maclean et al., 2000, Rizzo and
Kim, 2005). Some researchers even claim that motor
learning in virtual environments can surpass training
in the real world (e.g. Todorov et al., 1997).
Training in virtual environments also permits to
enhance the rehabilitation platform with
computational models and learning systems. In this
paper, we introduce a virtual adaptive biofeedback
rehabilitation approach aimed at improving
neuromuscular training using artificial neural
networks able to learn from biofeedback and to
168
Barzilay O. and Wolf A..
LEARNING FROM BIOFEEDBACK - Patient-specific Games for Neuromuscular Rehabilitation.
DOI: 10.5220/0003679801680174
In Proceedings of the International Conference on Neural Computation Theory and Applications (NCTA-2011), pages 168-174
ISBN: 978-989-8425-84-3
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

produce online new patient-specific virtual
physiotherapy missions. With the help of a motion
capture system and electromyograms (EMG), our
rehabilitation system tracks at any time the
kinematics of a subject and his muscle activation.
The subject is exposed, via a head mounted display
unit, to virtual tasks, which he is asked to perform.
After a calibration phase, a neural network is trained
to respond to the subject's biofeedback information,
based on the desired muscle activation and motions
prescribed by the physiotherapist. Once training is
complete, the network calculates a new trajectory as
biomechanical exercise, subjected to the previous
performance of the subject. This adaptive loop is
repeated continuously, resulting in an online
biofeedback-based adaptive virtual rehabilitation
system.
We exploit in this study the ability of artificial
neural networks to accurately model systems on
which little information is available or complex
systems where a computational model is preferred
over an explicit model based on arbitrary
assumptions and constraints. Biological systems
undoubtedly are the most difficult systems to model,
as they often involve several functional elements.
For instance, virtual upper-limb rehabilitation not
only involves motor learning and control of the
subject, but also his interpretation of the virtual
environment or his hand-eye coordination faculty.
Our research hypothesis is that an artificial neural
network can be utilized to model biological systems
by defining only the input and output signals to the
system. This contrasts with works aiming at building
sophisticated internal models of human motor
control (e.g. Kawato 1990, 1999). We validate our
hypothesis on a simple case of neuromuscular
rehabilitation. To the best of our knowledge, the
approach presented in this paper is novel and has no
antecedent in the literature. The results of this study
can encourage others in this field to further explore
the ability of learning systems to model human
functions by the means of biofeedback
instrumentation.
2 METHODS
2.1 Experimental Setup
Using the Vicon™ motion capture system
capabilities, we track the subject's motions in real
time and continuously gather kinematic data.
Markers are placed on the subject's body or on part
of it (we first focused our study on the arms). During
training, the subject is immersed in a virtual
environment in which he is shown floating targets
(Figure 1). The subject is then asked, for instance, to
follow the motions of a target with his pointing
finger (virtual ball application). His motions are
continuously recorded by the system while
following the virtual missions presented to him.
Moreover in order to record the subject muscular
activation, we place electromyograms sensors on
key muscles associated related to the motion.
Figure 1: Experimental Setup.
The user receives, in real time, augmented
feedback on his performance during training. For
instance in the virtual ball application, in which the
subject must continuously get his hand as near as
possible to the ball in motion, the feedback on the
distance to the target is provided in several manners.
On the virtual replica of the subject's hand is drawn
a ball, having the same radius as the virtual ball to
reach. The color of this ball changes with respect to
the distance to the target, according to a pre-defined
color code. The subject can also read his score rising
proportionally to the distance to the target. An
additional indication on the distance to the virtual
ball in the horizontal plane is provided by the
shadows of both balls, projected vertically onto the
virtual ground. Those indicators allow the user to
correct his gestures while performing the exercise.
For an improved accuracy yet, when the subject is
relatively close to the target, an additional feedback
is given to the subject, as the volume of a musical
background varies according to the distance to the
target. This last estimator offers the subject the
opportunity to perform fine-tuning on his hand's
position. The activation of a specific muscle is also
displayed as sweat drops coming out the virtual
sleeve, proportionally to the produced effort.
2.2 An Inverse Model of the Subject
The subject can be seen as a model receiving a
biomechanical exercise as an input and producing a
performance, that may include kinematic signals
LEARNING FROM BIOFEEDBACK - Patient-specific Games for Neuromuscular Rehabilitation
169
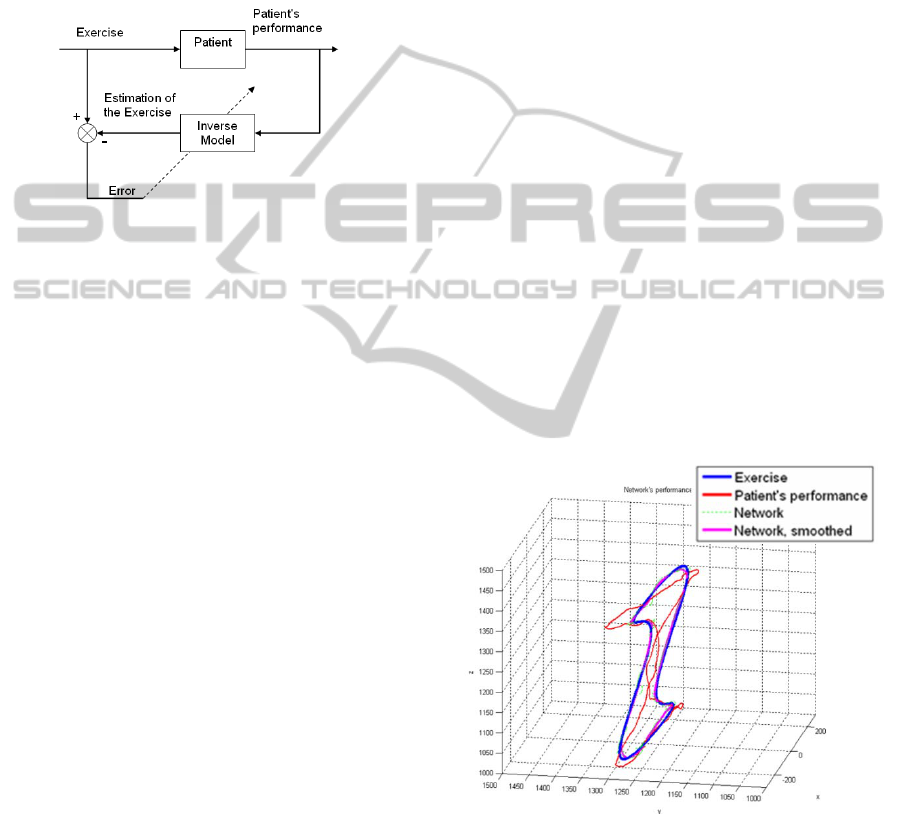
(e.g. the trajectory of a limb), as well as EMG
signals as an output. We wish to develop a system
able to generate a patient-specific physiotherapeutic
task, given the kinematic and/or muscular
performance of the subject. This goal may be
attained from an estimation of the inverse model of
the subject, with the desired performance as the
input and the exercise trajectory at the output
(Figure 2).
Figure 2: Best Estimated Inverse of the Subject.
2.3 The Learning System
Our goal is to train an artificial neural network
capable of producing a subject-specific
biomechanical task, given a desired subject’s
performance. The definition of the subject’s
performance is arbitrary and may include for
example kinematic, kinetic, or muscular parameters.
2.3.1 Network with Kinematic Input Only
In the first phase of our study, we tracked the
kinematics of the subject’s pointing finger. We use
this data to train a neural network. However, we first
have to determine the network architecture.
The universal approximation theorem for neural
networks states that every continuous function that
maps intervals of real numbers to an output interval
of real numbers can be approximated at any level of
desired accuracy by a multi-layer feed-forward
neural network with a single hidden layer having a
sigmoid activation function. In our case, the network
is designed to map, at each instant, the desired
position and velocity of a marker placed on the
subject, to another spatial point and velocity
corresponding to the displayed exercise.
Consequently, we need a system capable of
modeling the mapping from R
6
to R
6
. We assume the
mapping to be a continuous function and use the
approximation theorem to build a multi-layer feed-
forward network with one hidden layer. This
network has a generic architecture for all subjects
and is specific to this definition of the performance.
Nonetheless, each subject will have his own tuned
network.
To define the network’s architecture, we start
with a known exercise trajectory. The subject is
presented with this task and is asked to follow the
target trajectory displayed to him. Concurrently, the
tracking system records his motions at given
timestamps. The recorded kinematic data serves as
an input set to the network, while the corresponding
exercises displayed to him serve as target outputs.
We use Levenberg-Marquardt error back-
propagation learning method (Moré, 1977) so that
the error between the actual output of the network
and the target output is minimized. The network’s
weights are initialized according to Nguyen-
Widrow’s method (Nguyen & Widrow, 1990).
The network used for a single marker contains
six input neurons and six output neurons:
corresponding to the spatial position and velocity
vectors. Next, the number of neurons in the hidden
layer needs to be determined. On the one hand, this
parameter affects the runtime and should therefore
be minimized. On the other hand, it also influences
the system’s accuracy and balance between
precision and efficiency must be attained. We
performed a set of tuning experiments where several
healthy subjects were given a cyclic trajectory as a
task to follow while their motions were tracked
(Figure 3).
Figure 3: Three-dimensional task and subject’s
performance.
The network was trained on the training set
composed by the exercise and the average
performance over the number of cycles. We used
three criteria to evaluate the network’s performance
for each subject and exercise: the output error, the
positional output error and the average of the
NCTA 2011 - International Conference on Neural Computation Theory and Applications
170

positional output errors over each of the ten different
cycles performed by the subject. The average of the
output errors reflected the network’s ability to
extrapolate its results on samples that were not
directly included in the training set. After iteratively
testing different sigmoid activation functions for the
hidden layer of the network, numbers of epochs and
numbers of hidden neurons, the final configuration
of the network was determined.
The resulting network comprised seven hidden
neurons with the hyperbolic tangent as activation
function and the number of epochs was set to 50.
The reader is referred to previous publication
(Barzilay and Wolf, 2009) for a detailed explanation
on the setting of the network’s architecture.
2.3.2 Network with Kinematic and EMG
signals as Input
In the second step of this research, we added the
patient's biceps and triceps EMG signals to the input
of the network, such that the new exercise would be
designed with respect to the information on the
muscles of the subject as well as the knowledge on
the kinematic data of his limb.
The data provided by the electromyograms
contain useful information that can be deciphered by
signal processing. There are numerous ways
described in the literature to extract this information
from the EMG signal, including analysis in time or
frequency domains. We use the workflow described
in Hodges and Bui (1996) to compute the linear
envelope of the signal by processing it in the time
domain. The processed signal is needed at every
instant in our application and the processing time
has to be minimized, all the more since several
signals are needed simultaneously. We accelerated
this operation by using the processed signals from
the precedent instant and reduced the processing
time by approximately 96% (Barzilay and Wolf,
2011). This fast implementation allows providing
the subject with continuous visual feedback on his
own muscular performance during the training.
The same parameters that were described in
section 2.3.1 are used to evaluate the network, but
now in addition to the 3D curve, the desired EMG
performance specific to that trajectory should also be
designed. We therefore determined a few desired
cyclic trajectories for the limb of the subject and
recorded the EMG performances of a dozen of
healthy subjects. The average of this set of data is
then used as the desired EMG performance over a
specific trajectory, and fed as input to the neural
network together with the trajectory of the desired
kinematic performance.
The number of neurons in the hidden layer has
been set to 17, according to the evaluation criteria
which were previously used.
2.3.3 System Evaluation
The first network, described in section 2.3.1,
considers only the endpoint kinematics of the subject
and has obviously less physiotherapeutic interest
than the network involving the subject’s muscular
performance (section 2.3.2). Nevertheless, the
optimistic results (section 3.1 and Barzilay and
Wolf, 2009) suggested evidence of the feasibility of
modeling human motor control with neural networks
and brought us to expand the subject model to
include muscular performance as well.
From a therapeutic perspective, the muscles
activation of the patient is more significant than his
ability to accurately reproduce specific trajectories.
For that reason, we focus our efforts on minimizing
the error in the EMG performance, whereas the
kinematics error is considered more moderately.
Although the EMG signals are calibrated from
measurement of the maximal voluntary contraction
prior to the training, the signals’ amplitudes tend to
differ between different subjects. Furthermore, we
focus on the rhythmical patterns of the muscles more
than on the activation intensity. To do that, we
consider the error between the desired and actual
EMG performance in the frequency domain.
For the evaluation of the adaptive system, we
thus consider the root mean squared deviation, in the
frequency domain, between the desired EMG
performance and the smoothed EMG performance of
the subject. Each participant (n = 16) performed
motor training on two exercises: the patient-specific
exercise produced by the trained neural network
(adapted training), and a general exercise having for
trajectory the desired kinematic performance. The
latter resembles a standard physiotherapeutic session
where the physiotherapist demonstrates to the
patient, for example with his hand, the desired
gesture to reproduce (conventional training). The
primary criterion for the system evaluation was
defined as the ratio of the errors obtained in the
performances in both cases.
3 RESULTS
3.1 Network with Kinematic Input
The exercise trajectory, designed by the network,
LEARNING FROM BIOFEEDBACK - Patient-specific Games for Neuromuscular Rehabilitation
171
deviates by 15 millimeters per point in average from
the exact trajectory. This average deviation is
reduced to 3-5 millimeters per point when a
smoothing filter is applied to the trajectory produced
by the network. The network succeeds by such to
estimate the inverse model of the subject.
In Figure 3, one can see that, due to the relative
location between the planar target and the subject's
eye, the task (in blue) can be perceived as a
projection on a plane normal to the subject's line of
sight. Nevertheless, the neural network system
learned and corrected the projection, although far
from being a linear phenomenon.
Figure 4: The exercise projection: side (left) and front
(right) views.
Once trained, the network is capable of
generating a patient-specific exercise, given a
desired patient performance. The inverse model of
the patient can be evaluated by comparing the
measured performance of the subject with respect to
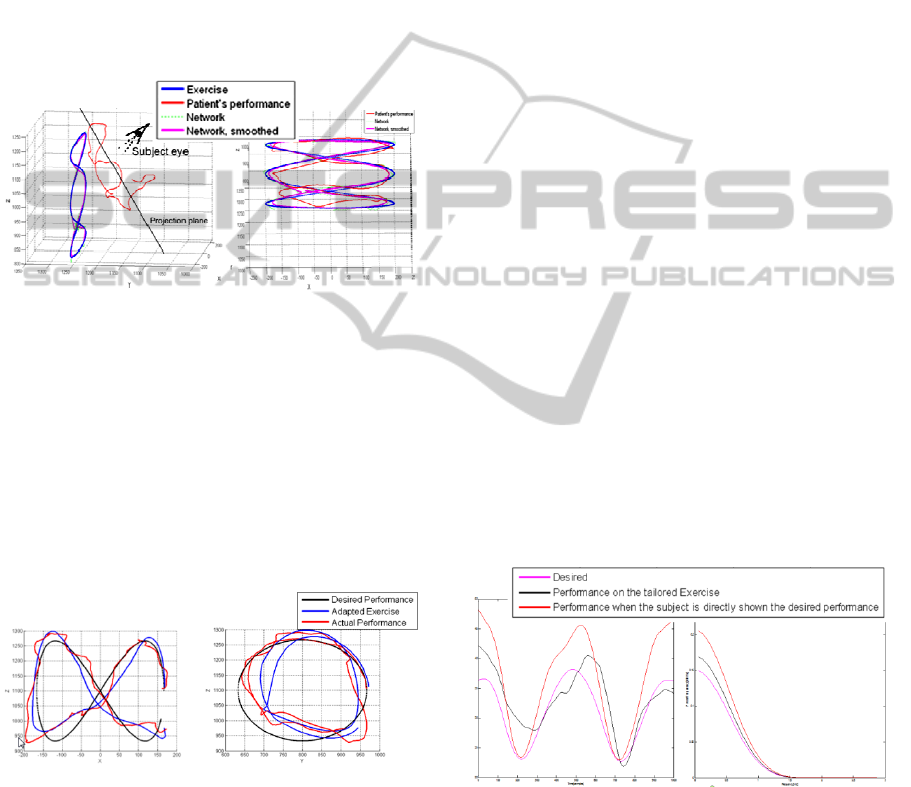
desired one. Given in figure 5 are the desired
performance, the patient-specific exercise created by
the network, and the performance of the subject on
this adapted task.
Figure 5: Desired Vs. actual performance: front (left) and
side (right) views.
Let us recall that, during rehabilitation session,
the subject does not see the whole exercise
trajectory, but only the virtual ball, in motion along
that trajectory. Moreover, he is not exposed to the
desired trajectory. In the depicted case, the average
distance between the subject's performed trajectory
and the desired performance was approximately
equal to the average distance between the hand
trajectory and the trajectory of the displayed
exercise. However, in several sections of the task,
the subject's trajectory was noticeably closer to the
desired trajectory than to the virtual ball, as can be
observed on the left side of the side view in Figure
5. It is also notable that, in this section, the network
was able to predict that, in order to cause the
subject's hand to follow the desired trajectory (in
black), the virtual ball had to be displayed a bit
farther along the y axis (farther from the subject).
This observation suggests that the model created by
the network was able to detect some of the subject's
behavioral patterns. This phenomenon was observed
in several sessions and for different subjects. It is
also notable, in Figure 5, that the system’s prediction
was effected twice on the same portion of the
exercise trajectory, while the subject’s hand had
different velocities.
3.2 Network with Kinematic and EMG
Signals as Input
Figure 6 demonstrates the capability of the network
to adapt itself to the muscular information recorded
from the electromyograms. Patterns characteristic to
the desired signal appear within the performance of
the subject on the exercise designed by the system.
Frequency-domain analysis shows how close the
spectra of the desired and the actual performance
signals are. We found that in many cases the subject
obtained a better performance on the network-
designed exercise than if he is directly shown the
desired trajectory of his limb as an exercise (Figure
6).
Figure 6: EMG performance: time (left) and frequency
(right) domains.
The deviations from the desired EMG
performance in the frequency domain for the
adapted and conventional training are presented in
Table 1. These results are summarized in Table 2.
NCTA 2011 - International Conference on Neural Computation Theory and Applications
172
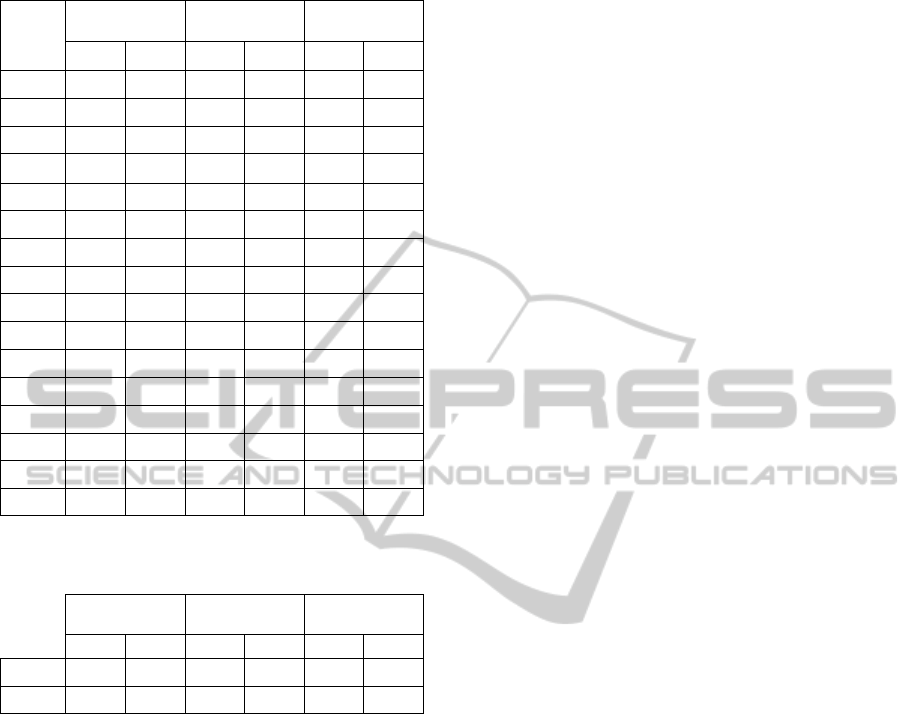
Table 1: Adapted and conventional training comparison.
Suhject
Adapted Training
Conventional
Training
Error Ratio
Biceps Triceps Bi. Tri. Bi. Tri.
#1
53.13 66.56 73.84 77.13 1.39 1.16
#2
36.25 85.10 76.95 79.76 2.12 0.94
#3
59.49 32.24 78.75 58.90 1.32 1.83
#4
85.00 85.64 40.44 92.74 0.48 1.08
#5
88.92 60.19 67.09 83.63 0.75 1.39
#6
63.85 97.61 56.58 84.20 0.89 0.86
#7
92.61 94.98 62.03 89.72 0.67 0.94
#8
62.94 39.51 18.53 68.19 0.29 1.73
#9
72.05 59.18 81.32 88.28 1.13 1.49
#10
74.67 42.19 148.20 58.98 1.98 1.40
#11
72.05 59.18 81.32 88.28 1.13 1.49
#12
65.00 29.28 74.27 86.06 1.14 2.94
#13
92.78 64.54 103.48 48.57 1.12 0.75
#14
56.12 55.39 71.95 69.94 1.28 1.26
#15
73.25 47.07 54.98 52.16 0.75 1.11
#16
44.71 93.98 54.85 72.04 1.23 0.77
Table 2: Adapted and conventional training comparison ˗
Summary.
Adapted Training
Conventional
Training
Error Ratio
Biceps Triceps Bi. Tri. Bi. Tri.
Average
68.30 63.29 71.54 74.91 1.10 1.32
Std. Dev.
16.50 22.53 28.10 14.16 0.48 0.54
Most subjects (n = 14, 87.5 %) benefitted from
our system for at least one of the muscles, and
almost half of them improved the accuracy of their
muscular performance for both biceps and triceps (n
= 7, 43.75%). In summary, the results indicate that
the average muscular performance of the subjects is
closer to the desired performance when the exercise
is generated by the system, rather than set as the
desired kinematic performance like in conventional
physiotherapy. This is indicated by a 10% increase
for the biceps performance and by 32% for the
triceps performance.
A one-tailed Student T-test shows that the
improvement in the triceps performance is attained
with statistical significance (p = 0.02). However, the
improvement in the biceps performance is lesser in
magnitude and in statistical significance (p = 0.34
and p = 0.09 with omission of two subjects). We
believe that this is due to the fact that the physical
exercise stimulated by the system involves the
biceps in a smaller measure than the triceps or the
shoulder muscles.
4 DISCUSSION
We introduce, in this study, a platform for motor and
cognitive rehabilitation, able to model the subject's
kinematics and to generate a subject-specific
physiotherapeutic exercise. The system requires no
prior knowledge on the patient, nor any model of his
motor control or trajectory planning. It only involves
the desired performance dictated by the
physiotherapist and a training reference set, recorded
in situ from the patient's performance prior to
rehabilitation. To date, and to the best of our
knowledge, no study combining virtual reality
rehabilitation and learning algorithms for patient-
specific training has been reported.
The developed system offers several
opportunities to both the physiotherapist and the
patient. The virtual tasks can be designed as
interactive games and stimulate the motivation of the
patient during rehabilitation. We have developed
several applications where the subjects are enjoined
to pop bubbles, stop soccer balls, or whack objects
with their hands in a controlled way. Most of the
participants expressed their enthusiasm after having
performed motor training in our virtual applications.
In every session, all the kinematic and EMG data
are stored and may be further analyzed offline by the
physiotherapist. Furthermore, the system proved to
emphasize some kinematic and muscular patterns in
motor training, and may contribute to a better
diagnosis of the subject. This system may find its
application in patients after stroke, with cerebral
palsy, dyslexia or other developmental coordination
disorders.
At this time, we have tested the system on
healthy subjects only, choosing to generalize and
validate it before testing it on pathological subjects.
The success of the system in learning some of the
subject’s behavioral patterns leads us to expect good
results in the modeling of motor patterns in patients
with pronounced pathology. Besides clinical trials,
we would like to expand the system to combine
more markers and EMG sensors, and other biometric
sensors.
Besides physiotherapy, this system could prove
useful in sportive performance enhancement, in the
development of new types of human-machine
interfaces, in entertainment, and in the training of
any kind of motor skills.
While a description of the subject model,
including his motor control and trajectory planning,
LEARNING FROM BIOFEEDBACK - Patient-specific Games for Neuromuscular Rehabilitation
173

hand-eye coordination and probably many additional
features, would be very difficult to elaborate, the
computational power of the simplest form of feed-
forward neural networks provided very optimistic
results in the modeling of the subject. First to
combine virtual rehabilitation with machine learning
of human models, the positive results of this study
encourage carrying on the use of biofeedback-based
artificial intelligence and virtual reality, for
applications in therapy and other diverse areas.
REFERENCES
Barzilay, O. and Wolf, A. (2009). An adaptive virtual
system for neuromuscular rehabilitation. In: IFMBE
Proc. World Congress on Medical Physics and
Biomedical Engineering, Munich, Germany. 25(4):
1291–94.
Barzilay, O. and Wolf, A. (2011). A fast implementation
for EMG signal linear envelope computation. Journal
of Electromyography and Kinesiology, 21: 678-682.
Hodges, P. and Bui B. (1996). A comparison of computer-
based methods for the determination of onset of
muscle contraction using electromyography.
Electroenc. Clin. Neurophysiol, 101, 511–519.
Holden, M. K. (2005). Virtual environments for motor
rehabilitation: review. Cyberpsychology & Behavior,
8(3),187–211.
Holden, M. K. and Dyar, T. (2002). Virtual environment
training: a new tool for neurorehabilitation. Neurology
Report, 26, 62–71.
Holden, M. K., Dyar, T., Schwamm L. and Bizzi, E.
(2005). Virtual Environment-Based Telerehabilitation
in Patients with Stroke. Presence: Teleoperators and
Virtual Environments, 14(2), April, 214-233.
Jack, D., Boian R., Merians, A. S., Tremaine, M., Burdea,
G. C., Adamovich, S. V., Recce, M. and Poizner, H.
(2001). Virtual reality–enhanced stroke rehabilitation.
IEEE Transactions on Neural Systems and
Rehabilitation Engineering, 9, 308–318.
Jacobson, J., Redfern, M. S., Whitney, S. L. and Wilson, J.
B. (2001). Balance NAVE: A Virtual Reality Facility
for Research and Rehabilitation of Balance Disorders.
In Proc. Virtual Reality Software and Technology
Meeting, Banff, Canada.
Kawato, M. (1990). Computational schemes and neural
network models for formulation and control of
multijoint arm trajectories. In Neural networks for
control, eds. Miller WT, Sutton RS and Werbos PJ.
MIT press.
Kawato, M. (1999). Internal models for motor control and
trajectory planning. Current Opinion in Neurobiology
9:718–727.
Maclean, N., Pound, P., Wolfe, C. and Rudd, A. (2000). A
qualitative analysis of stroke patients' motivation for
rehabilitation. BMJ, 321, 1051–1054.
Moré, J. J. 1977. The Levenberg-Marquardt algorithm:
Implementation and Theory. In: Numerical Analysis.
Lecture Notes in Mathematics 630, ed. Watson GA,
105–116. Berlin: Springer-Verlag.
Nguyen, D., Widrow, B. (1990). Improving the learning
speed of 2-layer neural networks by choosing initial
values of the adaptive weights. In Proc.International
Joint Conference on Neural Networks, July, 3:21–26.
Regian, J. W., Shebilske, W. L. and Monk, J. M. (1992).
Virtual reality: an instructional medium for visual
spatial tasks. Journal of Communication, 7, 131–145.
Rizzo, A. and Kim, G. J. (2005). A SWOT Analysis of the
Field of Virtual-Reality Rehabilitation and Therapy.
Presence: Teleoperators and Virtual Environments,
14(2), April, 119-146.
Shea, C. H. and Wulf, G. (1999). Enhancing motor
learning through external-focus instructions and
feedback. Human Movement Science, 18, 553–571.
Todorov, E., Shadmer, R. and Bizzi, E. (1997).
Augmented Feedback Presented in a Virtual
Environment Accelerates Learning of a Difficult
Motor Task. Journal of Motor Behavior, 29, 147–158.
VICON Motion Systems at http://www.vicon.com/
NCTA 2011 - International Conference on Neural Computation Theory and Applications
174
