
DUAL-ENERGY X-RAY ABSORPTIOMETRY AS AN
INDICATOR FOR FRAGILITY FRACTURE RISKS
OF THE FEMORAL NECK
Alexander Tsouknidas
1
, Nikolaos Michailidis
2
, Kleovoulos Anagnostidis
3
and Antonios Lontos
4
1
Laboratory for Machine Tools and Manufacturing Engineering, Mechanical Engineering Department
Aristoteles University of Thessaloniki, Thessaloniki, Greece
2
Physical Metallurgy Laboratory, Mechanical Engineering Department, Aristoteles University of Thessaloniki,
Thessaloniki, Greece
3
3rd Orthopaedic Department ”Papageorgiou” General Hospital, Aristoteles University of Thessaloniki,
Thessaloniki, Greece
4
Department of Mechanical Engineering, Frederick University, Nicosia, Cyprus
Keywords: Dual-energy x-ray absorptiometry, Femoral neck, FEM, Fragility fracture risks.
Abstract: Osteoporosis is a clinically silent bone pathology usually manifesting in the form of fragility bone fractures.
Due to the high morbidity of the disease, the association of noninvasive imaging techniques to the
implicated risk factors, could serve as a valuable indicator for surgeons. In the present investigations, the
evaluation of 30 patients femurs' bone mineral density was performed in vivo by Dual-energy X-ray
absorptiometry (DXA), while the strength characteristics of the examined specimens were determined ex-
vivo using uniaxial compression experiments. The obtained stress strain curves, reflect the mechanical
properties of the femur while facilitating their correlation to the obtained DXA measurements. FEM
simulations revealed critical stress values within the femoral neck, indicating which DXA values represent
abnormal high fragility fracture risks and thus should be considered for surgical intervention.
1 INTRODUCTION
Osteoporosis is a multifactorial bone disease con-
cerning roughly 4% of the human population
(Melton et al., 1992). As an asymptomatic condition,
osteoporosis fails to exhibit noticeable symptoms,
particularly at early stages and thus is usually
underdiagnosed. Untreated however, this clinically
silent disease, is likely to increase the risk of
fragility fractures (Ettinger, 2008); (Rockwood et al.,
1990); (Cooper et al., 1992). Due to its high
morbidity and global nature, osteoporosis is
considered a pathology with a significant
socioeconomic impact (Ray et al., 1997).
The affected patients' bone mineral density is
drastically reduced, deteriorating the bones'
micostructural characteristics as a result of excessive
bone resorption followed by insufficient bone
formation during remodeling (Frost and Thomas,
1963); (Raisz, 2005). The pathogenesis has been
associated to dietary aspects (Hackett et al., 2009),
immobilization (Minaire, 1989), hyper-
parathyroidism (Dupree and Dobs, 2004), vitamin D
deficiency (Holick, 2004), alteration of biochemical
markers like hormone (Parfitt et al., 1995); (Black et
al., 2003) and aging (Newton-John and Morgan,
1970). Regardless etiology, decreased bone mineral
density renders the skeletal system susceptibility to
fracture, predominantly occurring at the hip (Bohr
and Schaadt, 1985), the vertebral column (Old and
Calvert, 2004) and wrist (Dempster, 2011).
According to the World Health Organization,
osteopenia and osteoporosis are defined by the
patient's bone mass deviation, when compared to
that of an average, young and healthy adult(WHO,
1994) when measured by DXA.
Even though DXA can accurately determine the
minerals and lean soft tissue of the examined area,
the overall accuracy of the measurement is impaired
by the subtraction of the indirectly calculated fat
mass (St-Onge et al., 2004). Furthermore, DXA
results are represented as mass per area, thus not
considering the anisotropy of the bone tissue and are
96
Tsouknidas A., Michailidis N., Anagnostidis K. and Lontos A..
DUAL-ENERGY X-RAY ABSORPTIOMETRY AS AN INDICATOR FOR FRAGILITY FRACTURE RISKS OF THE FEMORAL NECK.
DOI: 10.5220/0003770000960101
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2012), pages 96-101
ISBN: 978-989-8425-90-4
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
hence as a quantitative and not qualitative index of
the bone structure(Lochmuller et al., 2000).
Several other methods have been recently
introduced to determine bone mineral density
(Genant et al., 1996, Braun et al.1998), DXA
nevertheless is still widely considered as the method
of choice, as techniques like peripheral quantitative
computed tomography (pQCT) may be accurate in
measuring BMD at peripheral skeletal sites, exhibit
however restrictions that prohibit measurements at
the proximal femur (Augat et al., 1996); (Augat et
al., 1998).
The aim of this investigation is to determine the
correlation of the bone mineral density in the
femoral neck, as measured by DXA, to
experimentally determined strength characteristics
of the bone. This, followed by the introduced FEM
simulation, will facilitate the use of DXA as an
indicator of fragility fracture risk in the hip region,
as there is a consensus throughout literature that hip
fractures involve the most severe consequences of
osteoporotic bone loss.
2 MATERIALS AND METHODS
This study was conducted on femoral neck samples,
harvested from patients undergoing total hip
replacement due to osteoarthritis. In order to
determine the samples' structural integrity, standard
X-rays (anterior- posterior) of the pelvis were taken
preoperatively in all cases. Patients with a sort
femoral neck, large cysts in neck region or previous
surgeries in proximal femur were excluded from the
study.
Overall 30 patients (27 female and 3 male) were
considered as representative candidates for this
study and thus subjected to DXA, to catalogue their
proximal femur bone mineral density. The average
age of these patients was 63.7 years (57- 76 years).
During the surgical procedure and after a 45
o
osteotomy, femoral heads were removed and stored
at -60oC until evaluation. A plane bone slice with
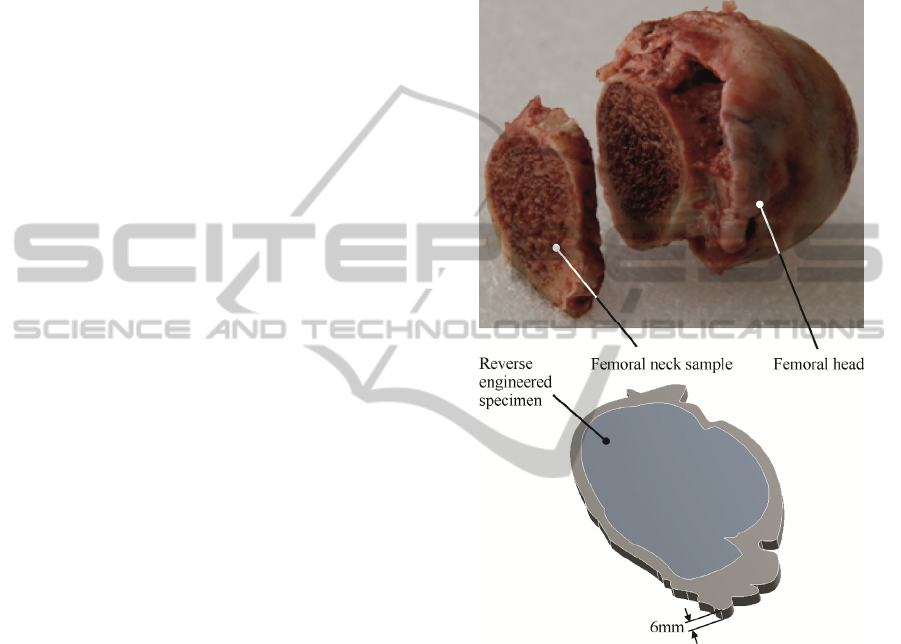
6mm thickness was harvested from the femoral neck
(see figure 1) as two parallel blades, mounted on a
mechanical saw at a 6mm distance, simultaneously
entered the proximal femur. This ensured similarity
among all specimens while producing parallel piped
specimens, directly employable in compression tests.
Mechanical testing was performed on an electric
INSTRON Testing system. To determine the
specimens’ strength characteristics, all samples were
subjected to uniaxial compression, until failure. A
cross-head traveling speed of 0.6mm/s was selected
and the maximum travelling distance (upon contact)
was set to 5mm in order to avoid contact of the
moving cross-head and the fixed base plate. To
reduce friction, the sample-actuator contact areas
were lubricated. The displacement of the cross-head
was measured by means of an inductive sensor, at an
accuracy of 1 µm.
Figure 1: Considered bone specimen and reverse
engineered model.
The biomechanical parameters were correlated
with BMD using the Pearson correlation coefficient
(r) and a linear regression model.
30 experiments were conducted to determine
both, compressive yield strength and modulus of
elasticity and associate these to the DXA determined
T-scores. The T-score compares the measured BMD
to that of a young adult (at the age of 35) of the same
gender with peak bone mass, while considering
statistical values.
3 RESULTS
A correlation of characteristic and mean values
DUAL-ENERGY X-RAY ABSORPTIOMETRY AS AN INDICATOR FOR FRAGILITY FRACTURE RISKS OF THE
FEMORAL NECK
97
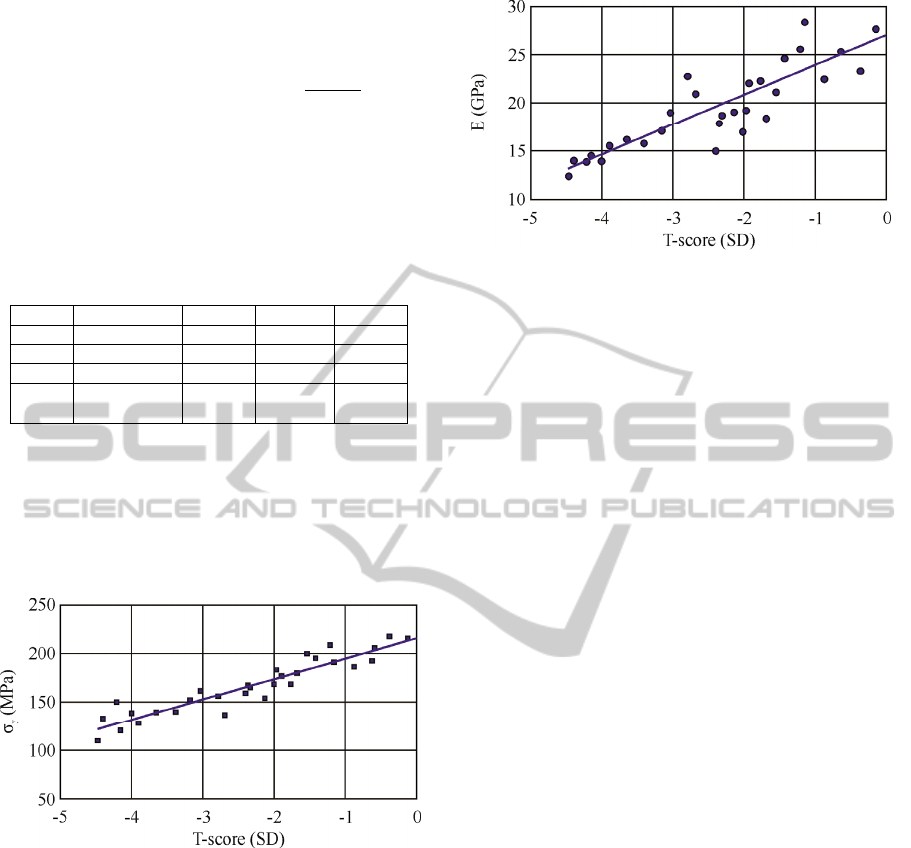
(BMD and T-score) determined by DXA
measurements, to the corresponding mechanical
properties (yield stress and elastic modulus) of the
examined specimens are reflected in table 1
. These
values are in good coherence with previously
presented data (Keller et al., 1990, Reilly and
Burstein, 1975). The offset in the determined values
can be attributed to the different sampling sites and
techniques of the compared studies.
Table 1: Descriptive values concerning BMD, T-score and
their correlation to the yield stress (σ
y
) and elasticity
modulus (E).
BMD (g/cm
2
) T-score σ
y
(MPa) E (GPa)
Min. 0.4638 -4.47 109.448 12.643
Max. 0.9694 -0.15 218.02 28.536
Mean 0.7248 -2.218 169.996 20.627
S.D. 0.263 24.843 4.129
A significant dependency of the femoral neck’s
yield stress and elastic modulus to the measured T-
score was affirmed. The highest correlation
coefficient was noted for T-score versus maximum
failure load (yield stress) of the samples (r=0.838,
p<0.001) as illustrated in figure 2.
Figure 2: Equivalent T-score values versus yield stress σy
(p<0.001).
A similar tendency can be observed for the
compressive moduli of the samples, which are
calculated based on the linear elastic region of the
determined stress-strain curves (Turner and Burr,
1993), as illustrated in figure 3.
A limitation however of the introduced process,
is based on the assumption of the material’s isotropy
and the determination of universal properties of a
bone segment comprising of both, cortical and
cancellous tissue. This methodology was adopted, as
DXA measurements reflect a combined BMA
encapturing both bone types by default and thus the
assumption of a compound material is beneficiary to
the approach.
Figure 3: Equivalent T-score values versus elasticity
modulus (p<0.001).
4 FEM SIMULATION
In order to associate the ultimate compression
strength of the samples, to fragility fracture risks of
the femoral neck, the geometry of the specimens was
reverse engineered and employed in a linear elastic
simulation of a gait type loading scenario
considering combined multiaxial forces (Jacobs et
al., 1997).
During the simulation, the specimens were once
again considered as a uniform-isotropic material,
comprising of cortical and cancellous bone tissue, to
directly facilitate the correlation of the DXA
measurements to the fracture risk of the femoral
neck.The experimentally determined mechanical
properties were adopted as bulk properties of the
compound material and assigned as such in the
simulation. The Poisson ratio was assigned as 0,3
corresponding to a mean value of cortical and
cancellous bone (Lu and Hutton, 1996, Smit et al.,
1997) regardless DXA value.
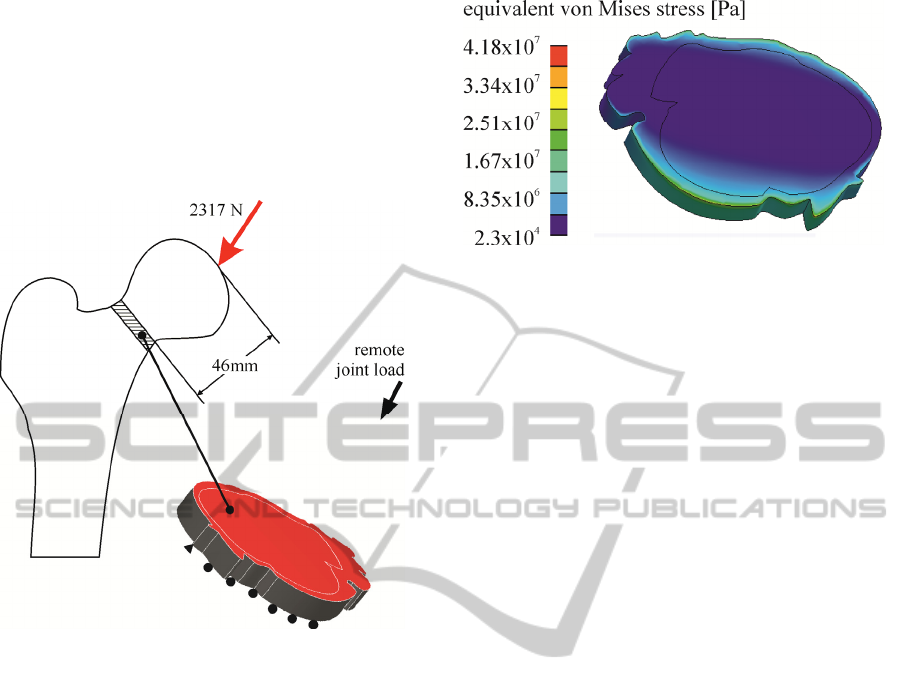
The acting loads on the femur, comprised of a
2317N joint force (Sarikat and Yildiz, 2011), evenly
distributed over the femoral head (inclined by 24
o
to
the frontal plane and 6
o
to the sagittal one). This
force was remotely applied on the upper surface of
the reverse engineered specimens at a distance of
46mm corresponding to the mean distance from the
tip of the femoral head at which the specimens were
severed from the femur. This, based on the
coordination system of the model, resulted in a
vector force comprising of Fx= 689N, Fy= 942N
and Fz= 2001N for axis x, y and z respectively.
The abductor muscle was considered as inactive,
as this muscle force acts during the lift up of the
foot, thus loading the trochanter during the
relaxation of the joint force. As the abductor muscle
force has been documented to amount to
approximately 703N, the worst case scenario during
BIOINFORMATICS 2012 - International Conference on Bioinformatics Models, Methods and Algorithms
98
normal loading of the femur, relates to the
aforementioned 2317N joint force.
The acting force and boundary conditions were
chosen to mimic the average loading history
encountered during walking of an adult human,
corresponding to 10.000 daily cycles as described by
Sarikat and Yildiz (2011) and are schematically
represented in figure 4.
Figure 4: Applied load and boundary conditions of the
developed FEM model.
There exists skepticism concerning the ability of
compression tests in predicting the hip fracture risk,
as fractures in the hip region are the effect of
complex dynamic force application, comprising of
shear, tension and compression. Based on the
forgoing description of the model, it becomes
evident that the conducted compression experiments
encapture the loading scenario in a realistic manner,
as the compressive strength of the femoral neck
exerts a dominant impact on the structural integrity
of the femur. Furthermore, the compression tests
were identically performed in all cases while the
only variation between samples was based on the
bone mineral density.
A characteristic stress field developing on a
femoral neck sample (T-score=-4.47,
σ
y
=109.448MPa and E=12.6GPa) is demonstrated in
figure 5.
5 DISCUSSION
DXA scans in the hip region, are conventionally per-
Figure 5: Calculated stress field on a reverse engineered
femoral neck sample.
formed in the trochanter, the Ward’s triangle and the
femoral neck (in an orthogonal area of6 by 10mm).
The aim of our study was to correlate the BMD
obtained from DXA, to the mechanical strength
characteristics of the examined area, as to provide
surgeons with a DXA based risk assessment,
concerning fragility fractures.
The introduced experimental investigation
affirmed the reliability of BMD in predicting the
mechanical properties of the femoral neck. A strong
enslavement of the ultimate material strength to
BMD (r=0.838) was found, while the correlation to
elastic modulus (r= 0.689) was weaker.
There exists a consensus throughout literature,
that bone density can be considered as a strong
independent predictor of failure strength
(Stankewich et al., 1996). Considering the foregoing
FEM simulation is based on static load representing
a daily cycle of at least 10.000 loadings, the
maximum developing stress should be multiplied by
3, to account for the fatigue safety factor. By
overlaying these fatigue stress value with the
experimentally determined fracture strength of the
examined specimens, a correlation between T-score
and fracture risk can be determined as demonstrated
in figure 6.
Even though DXA is a cost efficient BMD
determinant, dominating the preference of surgeons
due to its simplicity, there are some limitations
associated to the method that may affect the
accuracy of the introduced procedure.
As DXA quantifies the bone mass and not the
bone quality of a specific site, micro-fractures in
vertical trabeculae of cancellous bone will maintain
undetected. It is however widely accepted, that
micro-fractures exert an important influence on the
mechanical strength of the bone. Despite this, DXA
can be treated as a macroscopically indicator of bone
strength. Especially in the hip region, where gait like
loading ensures constant remodeling and thus the
DUAL-ENERGY X-RAY ABSORPTIOMETRY AS AN INDICATOR FOR FRAGILITY FRACTURE RISKS OF THE
FEMORAL NECK
99
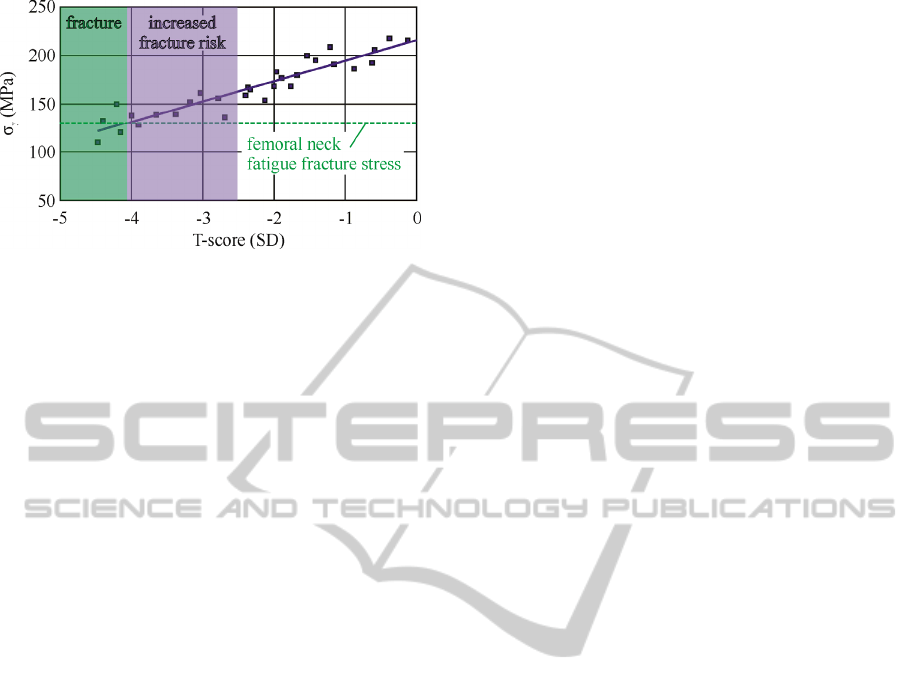
Figure 6: T-score as a fracture risk indicator.
probability of micro fractures is considered as rather
low.
Another possible limitation of our study is
associated to the patients, the samples were
harvested from, as all of them were diagnosed with
osteoarthritis. This might have a twofold effect on
the BMD-bone properties correlation.
Primary, it has not been established if the most
common musculoskeletal disorders of the elderly
(osteoarthritisand osteoporosis) may be treated as
independent, studies have shown that the presence of
one disease may act protective against the other
(Solomon et al., 1982, Cooper et al., 1991). The
effect however of this on the presented results, can
be neglected as the selected patients exhibited
significant differences in terms of BMD.
Secondary, osteoarthritis has been associated to
subchondral scleroses in femoral head; the femoral
neck and the trochander region however, are rarely
affected by the condition (Li and Aspden, 1997). In
order to circumvent this aspect, our methodology
considered DXA scansin femoral neck, trochanter
and Ward’s triangle and was determinedas reliable.
Additionally, osteoarthritic patients undergoing total
hip arthroplasty were the only group of patients from
whom, we could receive bone samples from the
femoral neck region.
Studies have indicated that the femur carries a
30% of the applied loads in the subcapital region,
while the base of the neck is subjected by 96% of the
total load (Lotz et al., 1995). This strengthens the
vital role of the femoral neck’s capacity to transmit
the compressive stress from the joint to the shaft of
the femur. Although the etiology of osteoporotic hip
fracture is complex and multifactorial(Melton and
Riggs, 1985, Greenspan et al., 1994), bone quality
is, without a doubt, a major risk factor.
6 CONCLUSIONS
Bone mineral density measured by DXA, regardless
limitations associated to the technique’s ability to
encapture bone quality, is a strong predictor of bone
strength in the femoral neck region. Supported by an
adequate FEM simulation, DXA may be regarded as
a valuable tool during the prediction of BMD
spectrums which present a significant risk of
fragility fractures.
REFERENCES
Melton 3rd, L. J., Chrischilles, E. A., Cooper, C., Lane, A.
W., Riggs, B. L., 1992. Perspective. How many
women have osteoporosis? Journal of Bone and
Mineral Research 7, 1005-10.
Ettinger, B., 2008. A personal perspective on fracture risk
assessment tools. Menopause: The Journal of The
North American Menopause Society 15(5), 1023-26
Rockwood, P. R., Horne, J. G., Cryer. C., 1990. Hip
Fractures: a future epidemic? Journal of Orthopaedic
Trauma 4, 388-96.
Cooper, C., Campion, G., Melton, L.J., 1992. Hip
fractures in the elderly: A word- wide projection.
Osteoporosis International 2, 285-9.
Ray, N. F, Chan, J. K, Thamer, M., Melton, L. J., 1997.
Medical expenditures for the treatment of osteoporotic
fractures in the United States in 1995: Report from the
National Osteoporosis Foundation. Journal of Bone
and Mineral Research 12, 24-35.
Frost, H. M., Thomas, C. C., 1963. Bone Remodeling
Dynamics. Springfield, IL.
Raisz, L., 2005. Pathogenesis of osteoporosis: concepts,
conflicts, and prospects. Journal of Clinical
Investigation 115 (12), 3318–25.
Hackett, E. S., MacLeay, J. M., Green, M., Enns, R. M.,
Pechey, C. L., Les, C. M., Turner, A. S., 2009.
Femoral Cortical Bone Mineral Density and
Biomechanical. Properties in Sheep Consuming an
Acidifying Diet. Nutrition and Metabolic Insights 1,
11-6.
Minaire, P., 1989. Immobilization osteoporosis: a review,
Clinical Rheumatology 8 (2), 95-103 .
Dupree, K., Dobs, A., 2004. Osteopenia and Male
Hypogonadism. Reviews in Urology 6(6), S30–4.
Holick, M. F., 2004. Vitamin D: importance in the
prevention of cancers, type 1 diabetes, heart disease,
and osteoporosis. American Journal of Clinical
Nutrition 79 (3), 362-71.
Parfitt, A. M., Villanueva, A. R., Foldes, J., Rao, D. S.,
1995. Relations between histologic indices of bone
formation: implications for the pathogenesis of spinal
osteoporosis. Journal of Bone and Mineral Research
10(3), 466-73.
Black, D. M., Greenspan, S. L., Ensrud, K. E., Palermo,
L., McGowan, J. A., Lang, T. F., Garnero, P.,
BIOINFORMATICS 2012 - International Conference on Bioinformatics Models, Methods and Algorithms
100

Bouxsein, M. L., Bilezikian, J. P., Rosen, C. J., 2003.
The effects of parathyroid hormone and alendronate
alone or in combination in postmenopausal
osteoporosis. The New England Journal of Medicine
349(13), 1207-15.
Newton-John, H. F., Morgan, D. B., 1970. The loss of
bone with age, osteoporosis, and fractures. Clinical
Orthopaedics and Related Research 71, 229-52.
Bohr, H., Schaadt, O., 1985. Bone mineral content of the
femoral neck and shaft: relation between cortical and
trabecular bone. Calcified Tissue International 37,
340-4.
Old, J. L., Calvert, M., 2004. Vertebral compression
fractures in the elderly. American Family Physician
69(1), 111-6.
Dempster, D. W., 2011. Osteoporosis and the burden of
osteoporosis-related fractures. American Journal of
Managed Care 17(6), S164-9.
World Health Organisation. Assessment of fracture risk
and its application to screening for postmenopausal
osteoporosis. Technical Report Series. Geneva: WHO,
1994.
Genant, H. K., Engelke, K., Fuerst, T., Gluer, C. C.,
Gramp, S., Harris, S. T., et al., 1996. Noninvasive
assessment of bone mineral and structure: State of the
art. Journal of Bone and Mineral Research 11, 707-30.
Braun, M. J., Meta, M. D., Schneider, P., Reiners, C.,
1998. Clinical evaluation of a high-resolution new
peripheral quantitative computerized tomography
(pQCT) scanner for the bone densitometry at the lower
limb. Physics in Medicine and Biology 43, 2279-94.
Augat, P., Reeb, H., Claes, L. E., 1996. Prediction of
fracture load at different skeletal sites by geometric
properties of the cortical shell. Journal of Bone and
Mineral Research 11, 1356-63.
Augat, P., Fan, B., Lane, N.E, Lang, T. F, LeHir, P., Lu,
Y., Uffmann, M., Genant, H. K., 1998. Assesment of
bone mineral at appendicular sites in females with
fractures of the proximal femur. Bone 22, 395-402.
St-Onge, M. P., Wang, J., Shen, W., Wang, Z., Allison, D.
B., Heshka, S., Pierson, R. N., Heymsfield, S. B.,
2004. Dual-Energy X-Ray Absorptiometry-Measured
Lean Soft Tissue Mass: Differing Relation to Body
Cell Mass Across the Adult Life Span. Journals of
Gerontology Series A: Biological Sciences and
Medical Sciences 59(8), 796-800.
Lochmuller, E. M., Miller, P., Burklein, D., Wehr, U.,
Rambeck, W., Eckstein, F., 2000. In situ femoral DXA
related to ash weight, bone size and density and its
relationship with mechanical failure loads of the
proximal femur. Osteoporosis International 11, 361-7.
Turner, C. H., Burr, D. B., 1993. Basic biomechanical
measurements of bone: a tutorial. Bone 14, 595-608.
Jacobs, C. R., Simo, J. C., Beaupre, G. S., Carter, D. R.,
1997. Adaptive bone remodeling incorporating
simountaneous density and anisotropy considerations.
Journal of Biomechanics 30(6), 603-13.
Keller, T. S., Mao, Z., Spengler, D. M., 1990. Young’s
modulus, bending strength and tissue physical
properties of human compact bone. Journal of
Orthopaedic Research 8, 592-603.
Reilly, D. T., Burstein, A. H., 1997. The elastic and
ultimate properties of compact bone tissue. Journal of
Biomechanics 8, 393-405.
Lu, Y. M., Hutton, W. C., Gharpuray, V. M., 1996. Do
bending, twisting and diurnal fluid change in the disc
affect the propensity to prolapse? A viscoelastic finite
element model. Spine 21, 2570-9.
Smit, T. H., Odgaard, A., Schneider, E., 1997. Structure
and function of vertebral trabecular bone. Spine, 22,
2823-33.
Sarikat, M., Yildiz, H., 2011. Determination of bone
density distribution in proximal femur by using the 3D
orthotropic bone adaption model. Proceedings of the
institute of Mechanical Engineering, Part H: Journal
of Engineering in Medicine 225, 365-75.
Stankewich, C. L., Chapman, J., Muthusamy, R., 1996.
Relationship of mechanical factors to the strength of
proximal femur fractures fixed with cancellous screws.
Journal of Orthopaedic Trauma 10, 248-57.
Solomon, L., Schnitzler, C. M., Browett, J. P., 1982.
Osteoarthritis of the hip: the patient behind the
disease. Annals of the Rheumatic Diseases 41, 118-25.
Cooper, C., Cook, P. L., Osmond, C., Cawley, M. I. D.,
1991. Osteoarthritis of the hip and osteoporosis of the
proximal femur. Annals of the Rheumatic Diseases 50,
540-2.
Li, B., Aspden, R. M., 1997. Material properties of bone
from the femoral neck and calcarfemorale of patients
with osteoporosis or osteoarthritis. Osteoporosis
International 7, 450-6.
Lotz, J. C., Cheal, E. J., Hayes, W. C., 1995. Stress
distributions within the proximal femur during gait
and falls: implications for osteoporotic fracture.
Osteoporosis International 5, 252-61.
Melton, L. J., Riggs, B. L., 1985. Risk factors for injury
after a fall. Symposium on falls in the elderly:
biological and behavioral aspects. Clinics in Geriatric
Medicine 1, 525-39.
Greenspan, S. L., Myers, E. R., Maitland, L. A., Resnick,
N. M., Hayes, W. C., 1994. Fall severity and bone
mineral density as risk factor for hip fracture in
ambulatory elderly. Journal of the American Medical
Association 271, 128-33.
DUAL-ENERGY X-RAY ABSORPTIOMETRY AS AN INDICATOR FOR FRAGILITY FRACTURE RISKS OF THE
FEMORAL NECK
101
