
A REGISTRATION FRAMEWORK FOR EVALUATION OF T1, T2
AND DWI SIGNAL INTENSITIES IN MULTIPLE MYELOMA
E. Montin
1
, P. Potepan
2
and L. T. Mainardi
1
1
Department of Bioengineering, Politecnico di Milano, Via Golgi 39, 20131 Milan, Italy
2
Dipartimento di Diagnostica per Immagini e Radioterapia, Fond. IRCCS Istituto Nazionale Tumori
Via Venezian 1, 20133, Milan, Italy
Keywords:
Whole Body Imaging, MRI, Myeloma.
Abstract:
Objective. In this study we point out the Diffusion-Weighted Imaging (DWI) role in the diagnosis of multiple
myeloma (MM), comparing its signal values (SV) (Sommer et al., 2010) with the standard imaging modalities
T1, T2. We further evaluate how SV change in relation with the percentage of plasma cells infiltration evalu-
ated through bone marrow biopsy (BMB).
Methods. Since March 2008 23 patients with average age of 61 (± 11) years old, 11 females and 12 males,
have been investigated before their own therapy with a whole body MRI protocol, concerning a whole body
T1, a whole body T2 and a whole body DWI and a BMB. An experienced radiologist defined for each patient
two volume of interests (VOIs): onto the main lesions and on healthy bones (Femur and Homerus). After that,
we have subdivided the full population by a clinical threshold of 25% on cells infiltration percentage; then, we
analysed statistical differences in the 2 groups (A, B).
Results. We found out that DWI voxels intensities in group A (infiltration ≤ 25%) were higher than group B,
this gap had to be considered statistically different (P ≤ 0.05).
1 INTRODUCTION
Although conventional radiography is still the stan-
dard widely approved staging procedure for newly di-
agnosed and relapsed multiple myeloma (MM) (Som-
mer et al., 2010), whole-body MRI (WB-MRI) using
T1- and T2-weighted contrast images has proved evi-
dence of its advantages over conventional skeletal sur-
vey (Ghanem et al., 2006). In this scenario the whole-
body DWI imaging is being attracting interest as a
tool for the investigation of MM lesions (Sakurada
et al., 2009). This study analyses the great potential
of DWI in MM diagnosis, comparing T1, T2, DWI
and correlating their values with clonal cells infiltra-
tion in bone marrow. When different MRI method-
ologies have to be compared, relevant role is plaid
by registration which allows the comparative analysis
among modalities. In this work we present a registra-
tion framework to align T1, T2 and DWI MRI acqui-
sition. The framework was designed for the evalua-
tion of signal intensities in Multiple Myeloma lesions.
2 MATERIAL AND METHOD
2.1 Patients
The analysed patients form a subset of a study
on whole-body MRI for multiple myeloma lesions
started in May 2008 at the national cancer institute
of Milan (INT). The study considers only patients at
diagnosis or at relapse after disease response (CR or
PR) lasting at least 6 months. The study was ap-
proved by the local ethics committee and all patients
gave their written informed consent before being in-
cluded. Globally, we analysed 23 patients (12 males
and 11 females, median age 61 years, age range 44, 81
years). Patients underwent a whole-body MRI scan
consisting of whole-body standard MRI (T1 and T2)
and Diffusion weighted MRI. None of the patients
had major artifacts on DW imaging that warranted
their exclusion from the study. After or immediately
before the MRI exams the patients underwent to bone
marrow biopsy (BMB).
563
Montin E., Potepan P. and Mainardi L..
A REGISTRATION FRAMEWORK FOR EVALUATION OF T1, T2 AND DWI SIGNAL INTENSITIES IN MULTIPLE MYELOMA.
DOI: 10.5220/0003777505630566
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (MIAD-2012), pages 563-566
ISBN: 978-989-8425-89-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
2.2 Imaging Protocol
MRI examinations were performed on a 1.5 T
MR imaging scanner Siemens Avanto (Erlangen-
Germany) using 6 array coils (head, neck, abdominal,
torso, pelvic and legs). Whole body images were cre-
ated composing different volumes: T1 and T2 were
acquired coronally in 3 - 5 steps, EPI DWI images
were acquired axially in 8 - 10 steps depending on
patients’ height.
The coronal T1-weighted tse2d1rr2 sequence was
performed with TR 550, TE 9.5, voxels size ∼ 1 x
5 x 1 mm, ∼ 1 mm gap, a field of view (FoV) of 500
x 500 mm, a matrix of ∼ 500 x 500 , two NEX, TA
2.40 minutes.
Coronal T2-weighted spcir3d1345ns sequence was
performed with TR 4000, TE 367, voxels size ∼ 1
x 5 x 1 mm, ∼ 1 mm gap, a FoV of 500 x 500 mm, a
matrix of ∼ 500 x 500 , two NEX, TA 4 minutes.
Axial Echo-planar DW imaging was performed with
4 b-values (50, 400, 800, 1000
s
mm
2
), ep2ddiff : TR
7900, TE 81, voxels size ∼ 2.5 x 2.5 x 6 mm, ∼ 1
mm gap, a FoV of 400 x 400 mm, a matrix of ∼ 160
x 160, two NEX, TA 3 minutes (table 1).
The total acquisition time was 30 min, chest and
abdominal T1 and T2 sequences were acquired dur-
ing breath-hold; all others sequences were acquired
during free breathing. No contrast agent was applied.
Table 1: Principal sequence features parameters.
Features T1 T2 DWI
Orientation Coronal Coronal Axial
TR 550 4000 7900
TE 9.5 367 81
Voxels size 1x5x1 1x5x1 2.5x2.5x6
2.3 Image Data Analysis
To evaluate different tissues property on different
MRI modalities we need to create 3D volumes and
register them among modalities. Registration of a
whole-body volume has many challenges such as the
huge size of the matrix, the anisotropy of the volume,
the breathing of the patients etc . . . . We propose a
registration framework which is explained in the next
section.
2.3.1 3D Reconstruction and Registration
Whole-body examination was obtained by partial
MRI scans of sub-volumes; the first step was to re-
build a unique 3D whole-body volume from the sub-
volumes acquired by T1 and T2 sequences. The sub-
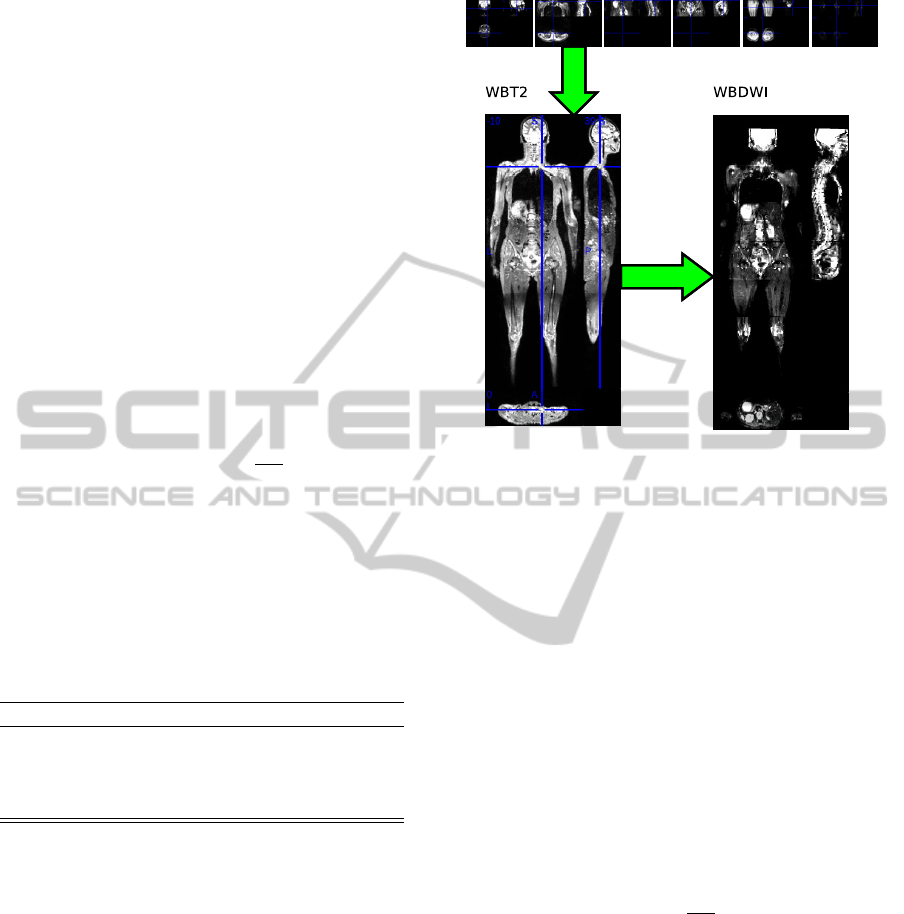
Figure 1: Reconstruction of whole-body DWI, each DWI
sub-volumes is aligned on the reconstructed whole-body
T2.
volumes were spatial combined and voxels averaged
in the overlapped areas. In addiction, to compensate
possible deformation due to patient breathing a non-
rigid registration between T1 and T2 was performed
using the T2 as reference imaging.
Conversely the 3D Volume reconstruction for the
DWI was obtained by the affine registration of each
DWI sub-volume with the reconstructed whole-body
T2. An affine registration was used in this purpose
and overlapped voxels were averaged. The proce-
dure is described by figure 1. To optimize compu-
tation time, the registration parameters were calcu-
lated for b50 volumes and then applied to the other
volume acquired with different b-value. Finally to re-
move possible local misalignment, a non-rigid regis-
tration was performed between whole-body T2 and
DWI at lowest b-value (50
s
mm
2
). All non-rigid reg-
istration were performed with IRTK software (Schn-
abel et al., 2001) using a multi-resolution optimiza-
tion with free-form deformations based on multi-level
B-splines (Lee et al., 1996) (Lee et al., 1997).
The total time for reconstruction and registration
was roughly 15 minutes for each patient. The accu-
racy of the registration was visually scored by an ex-
pert radiologist. None of the registered volume was
classified as non acceptable or erroneous by the radi-
ologist.
Our framework subdivides the registration task in
two parts: a global transformation and a local one.
This kind of solution is usually adopted in breast MR
Images to model the movement of the tissues (Rueck-
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
564
ert et al., 1999). The global motion model describes
the overall motion of the sub-volume and is com-
pensated by an affine transformation, which has 12
degrees of freedom, describing rotations and transla-
tions scaling and shearing.
For the local registration, through which we try to
compensate the local deformation of the sub-volume
(i.e. breathing movement on rib cage), we selected
a free form Deformations model (FFD), based on B-
splines (Rueckert et al., 1999). The basic idea of FFD
is to deform an object by manipulating an underlying
mesh of control points. The resulting deformation is
applied to the entire 3-D object and produces the final
transformation.
For both the models we used normalised mutual
information as voxels similarity measure and a work-
ing resolution of 5 x 5 x 5 mm for the global registra-
tion while a thinner mesh for the local model 2 x 2 x
2 mm.
2.3.2 Image Quantification
An experienced radiologist identifies for each patients
2 volumes of interest: within the main lesions (in the
surrounding of the BMB) and on healthy bone (Femur
and Homerus). The selection was performed in one of
the image modalities, usually on b1000 DWI images
as suggested by the literature (Khoo et al., 2011) and
easily transferred to the other as the volume was regis-
tered previously, averaged values inside the ROI were
computed for each image modality.
2.3.3 Statistical Analysis
Comparison between intensity of healthy bone and le-
sions was performed using a Wilcoxon’s signed-rank
test, the level of significance was set to P = 0.01.
In addition, the patients were categorised in two
groups based on the percentage of clonal cells in bone
marrow resulting from the BMB exam; group A had
an infiltration percentage ≤ 25% and group B > 25%.
An infiltration greater than 25% is for oncologist clin-
icians a typical threshold that decides for treatments
on patients. Mean values of the voxels intensities
were compared using Mann-Whitney rank-sum test,
to reveal the differences between the two groups, the
level of significance was set P = 0.05.
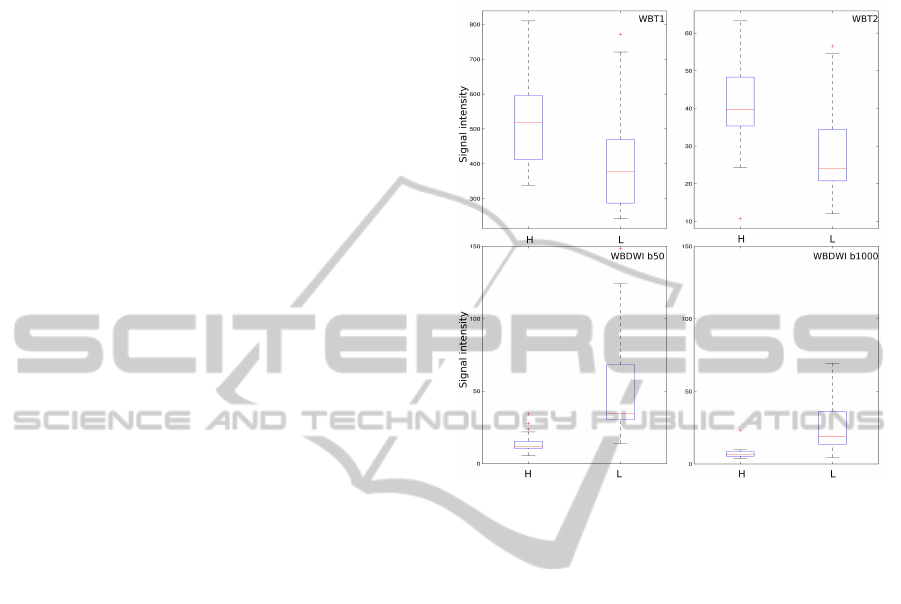
3 RESULTS
Median signal intensity and 25
th
and 75
th
percentile
are reported in table 2, the same data are shown in
box-plot of figure 2. In all the modalities a statisti-
cal significance P < 0.001 was observed between the
voxels values in the healthy ROI vs the lesions ROI.
Figure 2: Voxels intensity of the 2 ROI healthy bone mar-
row (H) and lesions (L) in T1, T2 and DWI (b50 ,b1000).
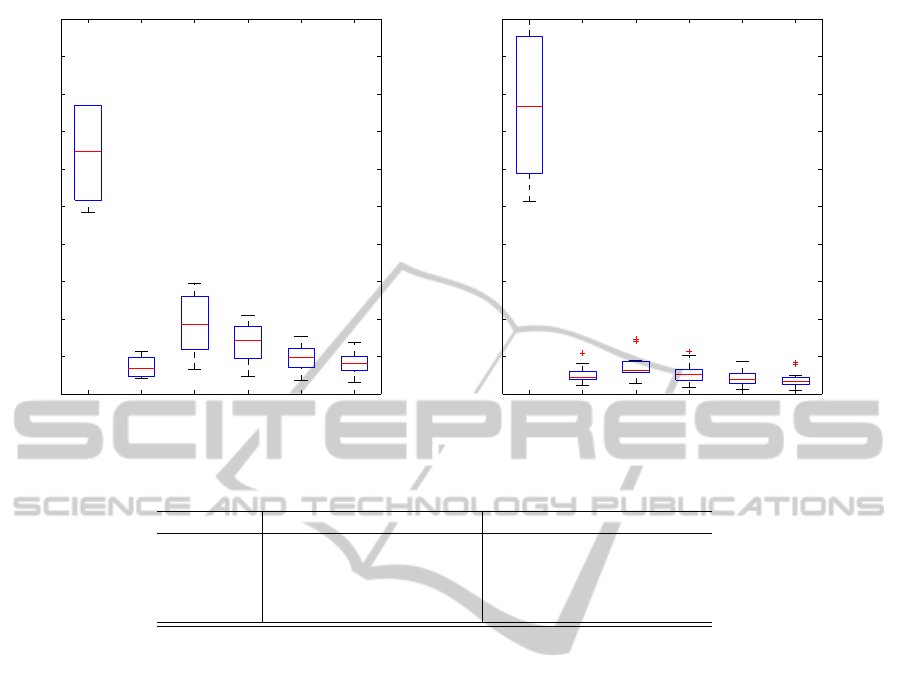
The second comparison between group A and
group B is shown in figure 3, it shows the difference
between the voxels values of the 2 groups (BMB≤
25% and BMB > 25%), significant difference is ob-
served between group A and group B in the all the
DWI imaging in particular in all the cases signal in-
tensity was lower in the group B (Koh and Collins,
2007) (Khoo et al., 2011). Conversely no significant
differences were observed in T1 and T2 imaging even
if a trend for lower value of lesions is observed.
4 CONCLUSIONS
Whole-body DWI imaging is a powerful tool for the
staging of patients with multiple myeloma (Padhani
et al., 2009) (Sommer et al., 2010). The new tech-
nique allows for fast whole-body imaging with low
technical and operational efforts. DWI sequences can
improve the accuracy of focal MM lesions identifica-
tion in patients newly diagnosed. A decrease of DWI
voxels values evaluated at the same b-value can be po-
tentially related to an high grade myeloma with cells
infiltration percentage higher than 25% (P ≤ 0.05).
A REGISTRATION FRAMEWORK FOR EVALUATION OF T1, T2 AND DWI SIGNAL INTENSITIES IN MULTIPLE
MYELOMA
565
WBT1 WBT2 DWITB50 DWITB400 DWITB800 DWITB1000
0
50
100
150
200
250
300
350
400
450
500
voxel value group A n 6
WBT1 WBT2 DWITB50 DWITB400 DWITB800 DWITB1000
0
50
100
150
200
250
300
350
400
450
500
voxel group B n 17
Figure 3: Ranksum test on T1, T2 and DWI (b50, b1000) voxels values in patient from group A and B.
Table 2: Median and quartile of lesion (L) and healthy bone marrow (H) pixel values of the different techniques.
techniques median
L
q 25
L
q 75
L
median
H
q 25
H
q 75
H
T1 397 287 470 517 412 596
T2 28.3 20.8 34.4 40.2 35.3 48.3
DWIb50 49.2 30.3 68.2 14.1 10.5 15.4
DWIb1000 23.7 13.5 36.2 7.31 5.36 8.49
REFERENCES
Ghanem, N., Lohrmann, C., Monika Engelhardtand Gre-
gor Pache, M. U., Saueressig, U., Kotter, E., and
Langer, M. (2006). Whole-body mri in the de-
tection of bone marrow infiltration in patients with
plasma cell neoplasms in comparison to the radiolog-
ical skeletal survey. Eur Radiol, 16:1005–1014.
Khoo, M., Tyler, P., Saifuddin, A., and Padhani, A. (2011).
Diffusion-weighted imaging (DWI) in musculoskele-
tal MRI: a critical review. Skeletal Radiology, pages
1–17.
Koh, D.-M. and Collins, D. J. (2007). Diffusion-Weighted
MRI in the body: Applications and challenges in on-
cology. Am. J. Roentgenol., 188(6):1622–1635.
Lee, S., Wolberg, G., and Shin, S. Y. (1997). Scattered
data interpolation with multilevel b-splines. IEEE
Transactions on Visualization and Computer Graph-
ics, 3:228–244.
Lee, S., Wolberg, G., yong Chwa, K., and Shin, S. Y.
(1996). Image metamorphosis with scattered feature
constraints. IEEE Transactions on Visualization and
Computer Graphics, 2:337–354.
Padhani, A. R., Liu, G., Koh, D. M. M., Chenevert,
T. L., Thoeny, H. C., Takahara, T., Dzik-Jurasz, A.,
Ross, B. D., Van Cauteren, M., Collins, D., Ham-
moud, D. A., Rustin, G. J., Taouli, B., and Choyke,
P. L. (2009). Diffusion-weighted magnetic resonance
imaging as a cancer biomarker: consensus and recom-
mendations. Neoplasia (New York, N.Y.), 11(2):102–
125.
Rueckert, D., Sonoda, L., Hayes, C., Hill, D., Leach, M.,
and Hawkes, D. (1999). Nonrigid registration us-
ing free-form deformations: application to breast mr
images. IEEE Transactions on Medical Imaging,
18(8):712–721.
Sakurada, A., Takahara, T., Kwee, T. C., Yamashita, T.,
Nasu, S., Horie, T., Cauteren, M. V., and Imai,
Y. (2009). Diagnostic performance of diffusion-
weighted magnetic resonance imaging in esophageal
cancer. Eur Radiol, 19:1461–1469.
Schnabel, J. A., Rueckert, D., Quist, M., Blackall, J.,
Castellano-Smith, A., Hartkens, T., Penney, G., Hall,
W., Liu, H., Truwit, C., Gerritsen, F., Hill, D.,
and Hawkes, D. (2001). A generic framework for
non-rigid registration based on non-uniform multi-
level Free-Form deformations. In Niessen, W. and
Viergever, M., editors, Medical Image Computing and
Computer-Assisted Intervention
ˆ
a MICCAI 2001, vol-
ume 2208 of Lecture Notes in Computer Science,
chapter 69, pages 573–581. Springer Berlin / Heidel-
berg, Berlin, Heidelberg.
Sommer, G., Klarh
˜
A¶fer, M., Lenz, C., Scheffler, K., Bon-
gartz, G., and Winter, L. (2010). Signal characteristics
of focal bone marrow lesions in patients with multi-
ple myeloma using whole body T1w-TSE, T2w-STIR
and diffusion-weighted imaging with background sup-
pression. European Radiology, pages 1–6–6.
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
566
