
INFERENCE ABOUT MULTIPLE PATHWAYS IN MOTOR
CONTROL LIMB IN LOCUST
C. D. Maciel
1
, D. M. Simpson
2
and P. L. Newland
3
1
Electrical Eng. Dept. - EESC, University of S
˜
ao Paulo, Av. Trabalhador S
˜
aocarlense 400, S
˜
ao Carlos, Brazil
2
Signal Processing and Control Group, University of Southampton
University Road Highfield, S017 1BJ, Southampton, U.K.
3
School of Biological Science, University of Southampton, University Road Highfield, S017 1BJ, Southampton, U.K.
Keywords:
Neuronal Analysis, Information Theory, Causal Inference.
Abstract:
In locust local circuits that control limb movements, the neural signals are processed by both spiking and
nonspiking interneurons that operate in parallel to process sensory information. These interneurons receive
sensory inputs from leg mechanoreceptors and together project to leg motor neuron pools. The main feature
of the nonspiking interneurons is their ability to communicate with other neurons without the intervention
of nerve impulses, or spikes, so that they exert graded control over their postsynaptic motor neurons, while
spiking local interneurons communicate by means of action potentials and are involved in the integration
of sensory signals. Our work presents an investigation from different classes of neurons driven by random
Gaussian excitatory movements to a proprioceptor at the knee joint. The underlying aim of this work was to
use information theory in understanding connectivity in the neural network.
1 INTRODUCTION
To improve the performance of robots many stud-
ies have focused on understanding how animals such
as insects (Fourtner, 1976) perform complex move-
ments using relatively simple neuromuscular reflex
control systems (Delcomyn, 2004). To produce a
reflex movement of a limb, the neuromuscular con-
trol system must transform an external stimulus into
a limb movement and in doing so must generate a
movement driven by appropriate neuronal patterns
(Burrows, 1996).
In many arthropods, and locusts in particular, it
is possible to perform measurements of the underly-
ing control signals (patterns of a neural activity) at
the level of the relatively few neurons responsible for
controlling movements of the legs. Locusts have a
distributed nervous system with a brain and a series
of segmental ganglion. Those in the thorax are re-
sponsible for generating the local movements of the
three pairs of legs (Burrows, 1996). The movements
of the tibia relative to the femur of a hing leg are mon-
itored and encoded by a sensory structure containing
approximately 90 sensory cells, the femoral chordo-
tonal organ (FeCO) that converts a mechanical stim-
ulus into electrical neuronal signals (Kondoh et al.,
1995). These sensory signals are processed in neu-
ronal networks containing different types of interneu-
rons that use both digital and analogue signalling to
control the activation of eleven excitatory motor neu-
rons (9 causing flexion and 2 causing extension) that
activate the tibial limb muscles to generate movement
of the tibia (Newland and Kondoh, 1997).
In a number of studies (Schreiber and Schmitz,
2000; Bialek et al., 2001; Ebeling, 2002) the idea that
a natural approach to analyse non-linear and stochas-
tic signals should be based on information theory has
been suggested. Information theory (Shannon, 1948;
Cover and Thomas, 2006) quantifies statistical un-
certainty in random processes and statistical depen-
dencies between multiple random processes (Cover
and Thomas, 2006). Entropy is a measure of uncer-
tainty of a random vector with a probability distribu-
tion function (pdf) p
X
(x) (Cover and Thomas, 2006;
Shannon, 1948). In addition, divergence is a gener-
alization of variance to processes with non-Gaussian
distributions (Cover and Thomas, 2006) while mutual
information is a measure of statistical dependency and
may be interpreted as a generalization of correlation
to arbitrary non-linear relationships between multiple
processes with arbitrary probability distributions (Er-
dogmus and Principe, 2006; Li, 1990).
69
D. Maciel C., M. Simpson D. and L. Newland P..
INFERENCE ABOUT MULTIPLE PATHWAYS IN MOTOR CONTROL LIMB IN LOCUST.
DOI: 10.5220/0003782200690075
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2012), pages 69-75
ISBN: 978-989-8425-89-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

The main limitation to mutual information is due
to its inability to differentiate the direction of associ-
ation between two signals (Schreiber, 2000). To over-
come this limitation (Schreiber, 2000) introduced a
quantity called information transfer that shares some
of the desired properties of mutual information but
takes into account the dynamics of information trans-
port. These quantities quickly spread to different
areas; from econometrics (Marschinski and Kantz,
2002) to biomedical engineering (Ward and Maza-
heri, 2008).
In general information theory approaches makes
few assumptions about the relationship among the
system variables, but does require strict stationarity
(Nichols, 2006). Time-delayed mutual information
quantifies co-dependence between variables by look-
ing at shared previous information content as a func-
tion of time lag (information flow) (Palu
ˇ
s et al., 2001;
Vastano and Harry, 1988).
Our study addresses the question of delayed
mutual information estimated from a large multi-
variate biological data set based on data used in
(Kondoh et al., 1995) and (Newland and Kondoh,
1997). Knowing the time-delay and information flow
strength between two signals will inform understand-
ing of the neural network function underlying limb
motor control in locusts. These insights and method-
ological developments are part of our wider interest in
neuromuscular (dys)function and bioinspired sensing
and control. The key challenge is how to combine the
recordings made in different animals into one map of
neuronal interconnections. In this experimental set-
ting (as is also often the case in related work), it is
not possible to record simultaneously from more than
perhaps two neurones, due to the physical size of the
preparation.
This investigation uses mechanical excitation by
random Gaussian displacements of a joint movement
sensor (the FeCO) to analysis of possible pathways
leading to electrical signal collected at different points
within the neural network, Fig. 1(C). In addition,
a surrogate test procedure (Schreiber and Schmitz,
1996; Schreiber and Schmitz, 2000) was used to infer
if any relationships were significantly above the noise
level. The final result represents a functional connec-
tivity map displayed using a graph tools to show the
time differences in neurophysiological measurements
along the processing chain, combining measurements
from many different recordings.
2 THEORY
Consider X = {x
i
} a discrete random signal. The en-
tropy H
X
is defined by (Cover and Thomas, 2006)
as H
X
= −
∑
x∈χ
p
X
(x)log
2
p
X
(x) where χ represents
the set of symbols used in this codification, p
X
(x) the
probability density function (pdf ) of event x, and H
X
quantifies the mean number of bits (when using base
two in log) that can optimally code random variables.
Entropy is a measure of disorder or more precisely un-
predictability. For example, systems with equiproba-
ble states have maximum entropy, since there is no
way to predict what will come next. A system that
could have many states but is held in just one particu-
lar state has zero entropy. Most data collections in the
real world lie somewhere between such extremes.
If p
Y
(.) is the pdf of variable Y , then
D
KL
[p
Y
kp
X
] =
∑
i
p
Y
(i)log
2
p
Y
(i)/p
X
(i) is the
Kullback-Leibler divergence (Cover and Thomas,
2006) quantifying the difference between p
Y
and p
X
.
It can be observed from the previous equations that
if p
Y
= p
X
then the D
KL
= 0. It should point out
that D
KL
[p
Y
kp
X
] 6= D
KL
[p
X
kp
Y
] and thus it is not
symmetric.
The mutual information from two random vari-
ables X and Y , I(X;Y ), quantifies the average shared
information or how the knowledge from one time
series informs about another (Cover and Thomas,
2006). This equation has been derived in many
references (Cover and Thomas, 2006; Shannon,
1948; Palu
ˇ
s and Vejmelka, 2007) I(X;Y ) = H(Y ) −
H(Y |X) = H(X) + H(Y) − H(X,Y) and expressed
from probabilities is:
I(X;Y) =
∑
x∈χ
∑
y∈φ
p
XY
(x,y)log
2
p
XY
(x,y)
p
X
(x)p
Y
(y)
(1)
where p
XY
(x,y) is the joint probabilities distribution
regarding X and Y , H(Y |X ) is the conditional en-
tropy of Y given X. Since H(Y ) ≥ H(Y|X) then
0 ≤ I(X,Y ) < ∞ (Dionisio et al., 2004), the equal-
ity is archived only when X and Y are indepen-
dent. From Eq. 1 it can easily be seen that
I(X ;Y ) = D
KL
[p(x,y)kp(x)p(y)] is the “distance”
from p
XY
(x,y) to p
X
(x)p
Y
(y), i.e. the assumption that
X and Y are independent.
The conditional entropy (Palu
ˇ
s and Vejmelka,
2007), H(Y |X), quantifies the remaining uncertainty
(entropy) of a random variable Y given another ran-
dom variable, X, and it is evaluated as:
H(Y |X) =
∑
x∈χ
∑
y∈φ
p
XY
(x,y)log
2
p
Y |X
(y|x). (2)
The conditional mutual information I(Y ; X |Z)
characterizes the dependence between X and Y with-
out the possible influence of another variable Z
(Cover and Thomas, 2006; Palu
ˇ
s and Vejmelka,
2007). This measure assesses the interaction X → Y
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
70

Figure 1: Diagram of the recording locations in the
metathoracic ganglion of the locust. The spiking local in-
terneurons were recorded from their somata on the ven-
tral midline of the metathoracic ganglion, whereas nonspik-
ing local interneurones were recorded in their neuropil pro-
cesses withing the area indicated by cross-hatching. Sen-
sory neurones were recorded in their axons in the ante-
rior half of nerve 5, while identified motor neurons were
recorded from their somata, the locations of which are
shown.
Table 1: Description of main neurones described in Fig. 1,
and used in signal acquisition.
Movement about knee:
ASFlTi Anterior Slow Flexor tibia motor neurone
AFFlTi Anterior Fast Flexor tibia motor neurone
AIFlTi Anterior Intermediate Flexor tibia motor neurone
PSFlTi Posterior Slow Flexor tibia motor neurone
PFFlTi Posterior Fast Flexor tibia motor neurone
PIFlTi Posterior Intermediate Flexor tibia motor neurone
SETi Slow Extensor Tibia motor neurone
FETi Fast Extensor Tibia motor neurone
Movement about ankle:
SleTa Slow Levator Tarsus
FleTa Fast Levator Tarsus
SDTa Slow Depressor Tarsus
FDTa Fast Depressor Tarsus
Movement of claw:
SRU Slow Retractor unguis motor neurone
FRU Fast Retractor unguis motor neurone
Movement about shoulder:
FLeTr Fast Levator Trochanter motor neurone
FDTr Fast Depressor Trochanter motor neurone
that is not only due to the parent random variable Z,
i.e. Z → X and Z → Y (Pearl, 2009).
Like mutual information, conditional
mutual information can be expressed as a
Kullback-Leibler divergence I(X;Y |Z) =
D
KL
[p(x,y,z)kp(x|z)p(y|z)p(z)] or as an ex-
pected value of simpler Kullback-Leibler
divergences: I(X ;Y |Z) =
∑
z∈Z
p(Z =
z)D
KL
[p(x,y|z)kp(x|z)p(y|z)].
(Nichols, 2006; Alonso et al., 2007) and others
have examined the structural dynamics of different
systems (the first study was on interactions from mus-
cle activity in pathological patient conditions and the
second used simulated mechanical system) based on
mutual information and conditional mutual informa-
tion.
Time delayed mutual information quantifies the
dependencies between dynamical variables by look-
ing at shared information content as a function of time
lags τ between X and Y . The delayed mutual informa-
tion that is (Nichols, 2006)
I(X,Y
τ
) =
∑
x∈χ
∑
y∈φ
p
XY
(x
i
,y
i+τ
)log
2
p
XY
(x
i
,y
i+τ
)
p
X
(x
i
)p
Y
(y
i+τ
)
. (3)
If the information present in signal X at a discrete
time i is also present in signal Y at discrete time i + τ
0
there will be a peak in the curve I(X,Y (τ)) at τ
0
> 0
as the joint probability density increases. If the peak
occurs at τ
0
< 0, that implies that the information is
being transported from Y to X (reverse order). At this
point it is assumed that X and Y are stationary and the
joint probabilities will depend only on the time lag τ
(Nichols, 2006).
The evaluation of interaction between random
variables X and Y has been presented in many articles
(e.g. (Schreiber, 2000; Palu
ˇ
s et al., 2001; Palu
ˇ
s and
Vejmelka, 2007; Palu
ˇ
s and Stefanovska, 2003)) and
is expressed using conditional entropies I(Y ; X
τ
|X)
(Nichols, 2006). Due to peculiarities of system topol-
ogy, Fig 1 (B,C) the approach adopted to analyse this
system is I(Y ; X
τ
|Z) where Z is the recorded mechani-
cal driver signal and τ varies from −T → T to include
analysis in both directions.
The data were also analysed to determine whether
they expressed any significant underlying dynamics
(Kugiumtzis, 2002). The validation procedure was
based on null hypotheses statistical tests for the ob-
served data (Urbach, 2000). To this end surrogate
data analysis was used (Schreiber and Schmitz, 1996;
Schreiber and Schmitz, 2000; Kugiumtzis, 2002).
The basic idea from the surrogate data analysis was
to compute the statistics of interest for the original
data set and for each of the ensemble of surrogate
data sets with equivalent amplitude distribution and
power spectra but forming independent from X, Y and
Z. If the computed statistics for the original data set
are significantly different from the values obtained for
the surrogate sets it is possible to infer that the output
data are related by the input signal.
For univariate time series the most common
method is the use of Fourier Transformation of the
data, randomising the phase and inverting the trans-
form. This removes any correlation between signals.
The surrogate data will have the same power spectrum
of the original and considering the Wiener-Khintchin
INFERENCE ABOUT MULTIPLE PATHWAYS IN MOTOR CONTROL LIMB IN LOCUST
71
theorem the same autocorrelation function. The liter-
ature presents many improvements on this algorithm
(Venema et al., 2006) in particular with reference to
amplitude distribution.
3 MATERIALS & METHODS
Adult male and female desert locusts, Schistocerca
gregaria (Forsk
˚
al) were used for all experiments. Lo-
custs were mounted ventral-side-uppermost in mod-
elling clay and the apodeme of the FeCO exposed by
opening a small window of cuticle in the distal ante-
rior femur (Kondoh et al., 1995), grasped between the
tips of fine forceps attached to a vibrator and cut dis-
tal to the forceps. The metathoracic ganglion was ex-
posed by making a small window in the ventral thorax
and removing air sacs and connective tissue. Micro-
electrodes with DC resistances of 50 − 80 MΩ were
driven through the sheath and into the neuropilar pro-
ceses of the spiking and nonspiking interneurons, Fig.
1.
The forceps holding the FeCO were moved with a
Gaussian white noise (GWN) signal produced by fil-
tering a pseudorandom binary sequence band-limited
to 27 Hz with a fourth order Chebyshev low-pass fil-
ter. Stimulus and evoked responses of the interneu-
rons were stored on magnetic tape using a PCM-DAT
data recorder. Subsequently, all signals were sampled
at a rate of 10 kHz offline to a PC for later analysis.
3.1 The Algorithms
The algorithms were developed in plain Python 2.67
running on a Linux (Ubuntu 10.10) i7 computer
with 12 GB of RAM. During the execution process
2 computers were used with the same specification
and communications base on SSH. The code used
mainly the resources of parallel programming from
the iphython (P
´
erez and Granger, 2007) environment.
The main libraries used in this work were: numpy,
scipy and matplotlib.
The data set consist of signals from 20 different
nonspiking local interneurons, 35 spiking local in-
terneurons, 20 sensory neurons and 34 identified mo-
tor neurons. All neuron pathways were stimulated for
at least 40s. We chose to analyse approximately 25
percent of each dataset where signals were approxi-
mately stationary (grey area in Fig. 2(a). The main
consequence of this long acquisition period is that
each analysed signal (discounting the transients and
zero input) has from 200.000 - 400.000 samples. For
regular signal analysis it took over 20 min to analy-
ses each file. Moreover, 35 repetitions of surrogate
(a)
(b)
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-150 -100 -50 0 50 100 150
200 Hz -> 27 Hz
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-150 -100 -50 0 50 100 150
27 Hz -> SETi
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-150 -100 -50 0 50 100 150
27 Hz -> FETi
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
-150 -100 -50 0 50 100 150
SETi -> FETi
(c)
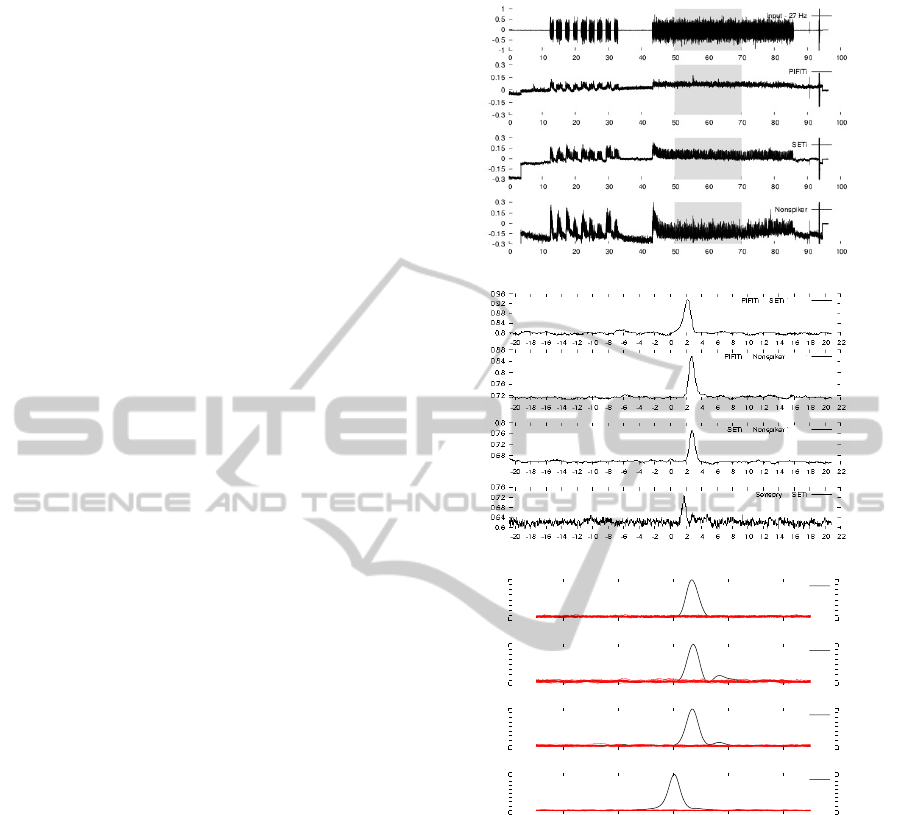
Figure 2: (A) Simultaneous intracellular recordings from
two motor neurons PIFlTi, SETI, and a nonspiker during
stimulation applied to the FeCO. The grey areas represents
the signal selection used in this analysis and the horizontal
axis is time in seconds. (B) Delayed Conditional Mutual
Information from combinations of signals shown in (A) and
the horizontal axis is delay between signals in milliseconds.
(C) This plot shows the delayed conditional mutual infor-
mation (line associate with the peak) between signal indi-
cated at the legend and delayed conditional mutual informa-
tion using surrogate date with 35 repetitions (baseline). The
vertical axis, left, has a normalized values and each mark
correspond to a step of 0.1.
data were also performed. The full analysis of this
data took approximately 35 days in a continuous par-
allel environment and at least one extra month was
required to analyse these initial results.
The estimation of probability density used a his-
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
72

togram approach, while for joint probability distribu-
tions bidimensional histograms were used. In both
cases the number of bins was fixed at 128. The de-
layed mutual information and conditional mutual in-
formation were implemented as a function of a lag.
Each lag was executed in a different core of the com-
puters.
4 RESULTS
For evaluation of the algorithms we used simulated
signals (with a linear and non-linear system with
Gaussian random input signals) and real experimen-
tal signals. In many signal records there were both
200 Hz and 27 Hz data. The time delays from 200 Hz
→ 27 Hz, Fig.2(c) first plot, agreed with time-delay
specifications of the low-pass filter used and when re-
versing input and output, 27 Hz → 200 Hz, the correct
negative lag were obtained.
Simultaneous recordings from interneurons and
motor neurons showed that they received either exci-
tatory or inhibitory inputs during stimulation applied
to the FeCO, Fig.2(a). This conclusion is based on
extensive previous work (Kondoh et al., 1995; New-
land and Kondoh, 1997; Vidal-Gadea et al., 2010) and
parallel work not further reported here.
We evaluated the time delay from recording sites,
1, considering the peaks of delayed mutual informa-
tion and delayed conditional mutual information. Dif-
ferent combinations of these sites were evaluated and
distinct peaks with relatively short delays were clearly
apparent (all graphics in Fig.2(b)). In Fig.2(b) (top
to down, three first graphics) the analyses was per-
formed with delayed mutual information and last plot
from delayed conditional mutual information where
the conditional was 27 Hz signal. Using this approach
we analysed the delayed (conditional) mutual infor-
mation from responses of sensory neurons, spiking
and nonspiking local interneurons and motor neurons
resulting from stimulation of the FeCO.
The results of our analysis are summarised in
Fig.3 in which the presumed interconnections based
on delayed (conditional) mutual information are
shown. The numbers on the arrows give the mean
time-delay (ms).
Some of recorded signal were collected at the
same place in neuronal ganglia from different ani-
mals. In this case, data were average and in few cases
outliers were removed from mean evaluation. In one
case (Nonspiker1 and Nonspiker2 in Fig.3) there were
enough data to cluster into two different sets, with dif-
ferent mean delay value and the graph was rerouted to
accommodate this split state. Also, in part of the data
there were signals with two peaks with similar am-
plitude time delay mutual information that were sta-
tistically significant. In these cases the peaks were
considered as representing different pathways.
The results reveal a number of important points.
First, that neurons sharing similar functions and that
are closely located in the network give similar time-
delays. For example, all identified flexor motor neu-
rons recorded from anterior and posterior groups, that
generate flexion movements of the tibia, have only
short delays of only a few milliseconds relative to
each other. Similarly, the two extensor motor neurons
(the slow, SETi, and fast, FETi) that produce exten-
sion movements of the tibia share information with
similar short delays.
Secondly, nonspiking interneurons can be parti-
tioned into two distinct groups (marked as nonspiker
1 and 2) with short and long time delays with respect
to the stimulus input, reflecting their potential roles
in the neural pathways controlling limb movements.
Third, while the dataset on which this analysis was
based was very large, it should be considered as a
small sample probing network characteristics. It is
impossible to record from all combinations of neu-
rons and so many potential interconnections are miss-
ing or have few repetitions. Additional recordings are
clearly needed for a more complete analysis of the
network.
Clearly it would be desirable to confirm the anal-
ysis with detailed anatomical studies of the neuronal
connections of the actual cells from which the data
was collected. However, this would be challenging
and was not carried out in this data set. In addition,
the connections with longer time-lags are unlikely to
be mono-synaptic, and such connectivities would be
difficult to disentangle from anatomical studies. Thus
while agreement between anatomy and the current
study would provide supportive evidence for the ap-
proach taken, disagreement would not necessarily im-
ply that the proposed signal processing method failed.
It is a strength of the current method that connec-
tions are identified based on information transmis-
sion, rather than direct neuronal connections.
It should also be pointed out that the current
method based on the simultaneous recordings from
only one or two neurons cannot clearly indicate if
connections are direct or indirect.
5 CONCLUSIONS
Previous work (Kondoh et al., 1995; Newland and
Kondoh, 1997) has analysed the anatomical connec-
tions between neurones, but such methods are not able
INFERENCE ABOUT MULTIPLE PATHWAYS IN MOTOR CONTROL LIMB IN LOCUST
73

Nonspiker
Nonspiker1
Nonspiker2
13.0
SETi
18.7
PSFlTi
20.5
AFFlTi
2.9 20.6
PFFlTi
3.1
ASFlTi
0.3
PIFlTi
2.2
2.1
FETi
2.5
2.7
0.31.7
2.8
2.7 2.5
4.6
1.7
2.6
MechInput
sensory
11.9
3.9
19.9 6.7
7.8
Spiking
6.0
Figure 3: Graph showing the presumed interconnections
based on delayed conditional mutual information analyses
with edge labels showing the mean time delays through
nodes in ms. The red edge labels indicate presumed con-
nections with incomplete data. Solid lines represent path-
ways that were evaluated directly from each dataset. Dotted
lines were inferred under the conditions that FeCO move-
ment activates sensory neurons whose time delays were not
included in the analyses. Dashed lines represent the parti-
tioning of nonspiking interneurons into two nodes.
to identify the activation of these connections during
the GWN stimulation used in the current study and
related work. The functional connections identified
in the current study broadly match those known from
the anatomical studies.
In previous work on this data we have used lin-
ear and non-linear system identification approaches
to study connectivity. However, this form of analy-
sis is limited by the class of models chosen (Volterra-
Wiener using polynomial non-linearities) whereas
the current information theoretic approach does not
impose such a constraints. Mutual information is thus
a better suited tool for mapping the connectivity in the
network. However, mutual information only shows
strength of connections (this will be considered in fur-
ther studies) and delays (considered here), but not de-
tailed input-output relationships.
The analysis of this data was realized using a com-
puter with multiple cores and with no downsampling
of the data. The approach was computationally inten-
sive and without a multiple core computer and a high
performance computational environment could not be
complete within a reasonable time. A key feature of
our analysis is that it can be used to understand the
interconnections between neurons in neural networks
composed of both spiking (digital action potentials)
and nonspiking (analogue synaptic) signals.
We found that neurons that share similar func-
tions, for example flexor or extensor motor neurons
that drive muscle activity (Newland and Kondoh,
1997) share similar time delays with respect each
group indicating their combined activity in control-
ling movements. Knowledge such as this can help
further understand the structure of neural pathways
within local circuits and can help inform further neu-
rophysiological analysis
(Burrows, 1996) suggested that the interactions
between nonspiking interneurons could lead to them
acting in many different ways to process the signals
from the FeCO. We found that a subset (4 and 6 mea-
surements of 14) of a population of nonspiking local
interneurons showed two distinct delayed mutual in-
formation time delays. Also, the last 4 measures from
nonspinking had 2 peaks with similar delays from 2
sets described before. This revealed itself in distinct
peaks in their responses indicating the presence of two
main pathways to the same neurone and points to dif-
ferences in the function of the two types of interneu-
ron in local networks, a feature not documented from
previous neurophysiological analyses.
The next steps in our study will be to include more
experimental samples in data analysis. The data anal-
ysis will also include an analysis of transfer entropy
and a Bayesian model to group partial graph informa-
tion.
ACKNOWLEDGEMENTS
The first author is indebted to the Research Founda-
tion of Brazil (CNPq) for support this collaboration.
We are also grateful to the BBSRC (UK) and EPSRC
(UK) for financial support.
REFERENCES
Alonso, J. F., Mananas, M. A., Topor, D. H. Z. L., and
Bruce, E. N. (2007). Evaluation of respiratory mus-
cles activity by means of cross mutual information
function at different levels of ventilatory effort. IEEE
Trans. on Biomedical Eng., 54(9):1573–1582.
Bialek, W., Nemenman, I., and Tishby, N. (2001). Pre-
dictability, complexity and learning. Neural Comp,
(13):2409 – 2463.
Burrows, M. (1996). The Neurobiology of an Insect Brain.
Oxford University Press, USA.
Cover, T. M. and Thomas, J. A. (2006). Elements of Infor-
mation Theory (Wiley Series in Telecommunications
and Signal Processing). Wiley-Blackwell, second edi-
tion.
Delcomyn, F. (2004). Insect walking and robots. Annu. Rev.
Entomol., 49(3):51–70.
Dionisio, A., Menezes, R., and Mendes, D. A. (2004). Mu-
tual information: a measure of dependency for nonlin-
ear time series. Physica A, 344:326–329.
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
74

Ebeling, W. (2002). Entropies and predictability of nonlin-
ear processes and time series. In Sloot, P., Hoekstra,
A., Tan, C., and Dongarra, J., editors, Computational
Science ICCS 2002, volume 2331 of Lecture Notes in
Computer Science, pages 1209–1217. Springer Berlin
/ Heidelberg.
Erdogmus, D. and Principe, J. C. (2006). From linear
adaptative filtering to nonlinear information process-
ing. IEEE Signal Processing Magazine, 14.
Fourtner, C. (1976). Central nervous control of cockroach
walking, pages 401–418. Plenum, New York.
Kondoh, Y., Okuma, J., and Newland, P. L. (1995). Dy-
namics of neurons controlling movements of a locust
hind leg: Wiener kernel analysis of the responses of
proprioceptive afferents. Journal of Neurophysiology,
73(5):332–340.
Kugiumtzis, D. (2002). Surrogate Data Test on Time Se-
ries, chapter 12, pages 267–282. Kluwer Academic
Publishers.
Li, W. (1990). Mutual information functions versus corre-
lation functions. Journal of Statistical Physics, 60(5-
6):823 – 837.
Marschinski, R. and Kantz, H. (2002). Analysing the infor-
mation flow between financial time series. Eur. Phys.
J. B, 30(2):275–281.
Newland, P. L. and Kondoh, Y. (1997). Dynamics of neu-
rons controlling movements of a locust hind leg ii.
flexor tibiae motor neurons. Journal of neurophysi-
ology, 77(3):1731–1746.
Nichols, J. (2006). Examining structural dynamics using in-
formation flow. Probabilistic Engineering Mechanics,
21(4):420–433.
Palu
ˇ
s, M., Kom
´
arek, V., Hrn
ˇ
c
´
ı
ˇ
r, Z., and
ˇ
St
ˇ
erbov
´
a, K. (2001).
Synchronization as adjustment of information rates:
Detection from bivariate time series. Phys. Rev. E,
63(4):046211.
Palu
ˇ
s, M. and Stefanovska, A. (2003). Direction
of coupling from phases of interacting oscillators:
An information-theoretic approach. Phys. Rev. E,
67(5):055201.
Palu
ˇ
s, M. and Vejmelka, M. (2007). Directionality of cou-
pling from bivariate time series: How to avoid false
causalities and missed connections. Phys. Rev. E,
75(5):056211.
Pearl, J. (2009). Causal inference in statistics: An overview.
Statistics Surveys, (3):96–146.
P
´
erez, F. and Granger, B. E. (2007). IPython: a System for
Interactive Scientific Computing. Comput. Sci. Eng.,
9(3):21–29.
Schreiber, T. (2000). Measuring information transfer. Phys.
Rev. Lett., 85(2):461–464.
Schreiber, T. and Schmitz, A. (1996). Improved surrogate
data for nonlinearity tests. Phys. Rev. Lett., 77:635.
Schreiber, T. and Schmitz, A. (2000). Surrogate time series.
Physica D, 142:346–382.
Shannon, C. E. (1948). A mathematical theory of communi-
cation. Bell System Technical Journal, 27(3):379–423.
Urbach, R. M. A. (2000). Footprints of Chaos in the Mar-
kets: Analyzing Non-linear Time Series in Financial
Markets and other Real Systems. Prentice Hall Pub-
lishing, USA.
Vastano, J. A. and Harry, S. L. (1988). Information trans-
port in spatiotemporal systems. Phys. Rev. Lett.,
60(18):1773–1776.
Venema, V. K. C., Ament, F., and Simmer, C. (2006). A
stochastic iterative amplitude adjusted fourier trans-
form algorithm with improved accuracy. Nonlinear
Processes in Geophysics, 13(3):247–363.
Vidal-Gadea, A., Jing, X. J., Simpson, D. M., Dewhirst, O.,
Kondoh, Y., Allen, R., and Newland, P. (2010). Cod-
ing characteristics of spiking local interneurons dur-
ing imposed limb movements in the locust. Journal of
Neurophysiology, 103:603 – 615.
Ward, B. D. and Mazaheri, Y. (2008). Information transfer
rate in fmri experiments measured using mutual in-
formation theory. Journal of Neuroscience Methods,
167(1):22–30.
INFERENCE ABOUT MULTIPLE PATHWAYS IN MOTOR CONTROL LIMB IN LOCUST
75
