
BAYESIAN-BASED EARLY DETECTION OF COGNITIVE
IMPAIRMENT IN ELDERLY USING fNIRS SIGNALS DURING
COGNITIVE TESTS
Shohei Kato
1
, Hidetoshi Endo
2
and Yuta Suzuki
3
1
Dept. of Computer Science and Engineering, Nagoya Institute of Technology
Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan
2
Dept. of Comprehensive Geriatic Medicine, National Center for Geriatics and Gerontology
35 Gengo, Morioka-machi, Obu, Aichi 474-8511, Japan
3
Dept. of Computer Science and Engineering, Nagoya Institute of Technology, Nagoya, Japan
Keywords:
Early detection of dementia, Functional near infrared spectroscopy, fNIRS, Bayesian classifier.
Abstract:
This paper presents a new trial approach to early detection of dementia in the elderly with the use of functional
brain imaging during cognitive tests. We have developed a non-invasive screening system of the elderly with
cognitive impairment. In addition of our previous research of speech-prosody based data-mining approach, we
had started the measurement of functional brain imaging for patient having a cognitive test by using functional
near-infrared spectroscopy (fNIRS). We had collected 42 CHs fNIRS signals on frontal and right and left
temporal areas from 50 elderly participants (18 males and 32 females between ages of 64 to 92) during cogni-
tive tests in a specialized medical institute. We propose a Bayesian classifier, which can discriminate among
elderly individuals with three clinical groups: normal cognitive abilities (NL), patients with mild cognitive
impairment (MCI), and Alzheimer’s disease (AD). The Bayesian classifier has two phases on the assumption
of screening process, that firstly checks whether a suspicion of the cognitive impairment (CI) or not (NL) from
given fNIRS signals; if any, and then secondly judges the degree of the impairment: MCI or AD. This paper
also reports the examination of the detection performance by cross-validation, and discusses the effectiveness
of this study for early detection of cognitive impairment in elderly subjects. Consequently, empirical results
that both the accuracy rate of AD and the predictive value of NL are equal to or more than 90%. This suggests
that proposed approach is adequate practical to screen the elderly with cognitive impairment.
1 INTRODUCTION
It is no doubt about abrupt increase in elderly patients
with dementia due to growing super-aging society in
developed countries. Research and development of
new dementia medications is accelerated. Develop-
ment of the early detection methods for dementia that
are both sensitive and specific is also very important
as a diagnostic tool.
To screen for dementia and cognitive impair-
ment, a questionnaire test such as Mini-Mental State
Examination (MMSE) (Folstein et al., 1975), Re-
vised Hasegawa’s Dementia Scale (HDS-R) (Imai and
Hasegawa, 1994), Clinical Dementia Rating (CDR)
(Morris, 1993), and Memory Impairment Screen
(MIS) (Buschke et al., 1999), is commonly used in
addition to a neurophysiological test (Zhang et al.,
2011) (e.g., using MRI (de Leon et al., 2004), FDG-
PET (Mosconi et al., 2010), and CSF biomark-
ers (de Leon et al., 2007)). Questionnaire tests have
some disadvantages and their use is limited in the
clinic. The MMSE, HDS-R, and CDR are more time-
consuming than a general practitioner’s consultation.
In general, the questionnaire cannot completely dis-
miss the influence of education, social class, and gen-
der difference on the results. In addition, there is a
possibility that practitioner subjectivity may affect the
scoring. Thus, we believe that the development of a
simple, non-invasive examination that is objectiveand
combined with a physiological test could enables the
early detection of dementia in a broad population.
In our previous study, we have studied a novel ap-
proach to the early detection of cognitive impairment
in the elderly (Kato et al., 2011), in which we fo-
cused on the prosodic features of speech sound dur-
ing the subject’s answers to the questionnaire. The
method had an advantage that enables everyone to
check his/her own cognitive ability anywhere because
118
Kato S., Endo H. and Suzuki Y..
BAYESIAN-BASED EARLY DETECTION OF COGNITIVE IMPAIRMENT IN ELDERLY USING fNIRS SIGNALS DURING COGNITIVE TESTS.
DOI: 10.5220/0003790001180124
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2012), pages 118-124
ISBN: 978-989-8425-89-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

Table 1: A Breakdown list of participants (N=50).
Age 64-70 71-75 76-80 81-85 86-92 Total
Male 3(2,0,1) 2(1,1,0) 4(3,1,0) 7(1,4,2) 2(0,0,2) 18(7,6,5)
Female 7(4,2,1) 7(5,2,0) 8(2,5,1) 6(2,1,3) 4(1,3,0) 32(14,13,5)
Subtotal 10(6,2,2) 9(6,3,0) 12(5,6,1) 13(3,5,5) 6(1,3,2) 50(21,19,10)
Value in bracket means the number of subjects in NL, MCI, AD clinical groups.
300sec 300sec
HDS-R test
60sec
rest
60sec
Reminiscence1
60sec
rest
60sec
Reminiscence2
60sec 60sec
Working Memory1
60sec
60sec
60sec
rest
60sec
Face recall
Listening Talking
Reminiscence3
Watching
60sec
rest
60sec
rest
Category recall
rest
Working Memory2
Reading span
Working Memory3
30sec
rest
Talking about hometown,
childhood, and school
00:00:00 00:14:00
00:10:00
00:14:00 00:22:30
timeline
timeline
Figure 1: Block design task of cognitive tests.
of using speech signals only. The method is effec-
tive for the first step of screening for dementia, but,
however, it has limitations of the reliability because
the method does not measure brain function. On the
other hand, a neurophysiological test, such as using
MRI, FDG-PET, and CSF biomarkers, imposes severe
constraint on a subject, for instance, pain at obtain-
ing cerebral spinal fluid, radiation exposure, physical
restraint and so on. This is a disadvantage in early
screening, which should covers all elderlies.
In this study, we focus on functional near-infrared
spectroscopy (fNIRS) as a brain function measure-
ment system, which can eliminate physical restraint
from a subject by non-invasive procedures, and de-
velop a prototype for computer-aided diagnosis of
cognitive impairment in the elderly with the use of
fNIRS signals during cognitive tests. In this paper,
we present signal processing technique of feature ex-
traction and selection for hyper-dimensional time se-
ries data of fNIRS signals, and propose the two-phase
Bayesian classifier for discriminating among elderly
individuals with three clinical groups. In addition,
we addressed the effectiveness of proposed method in
discriminating among elderly individuals with normal
cognitive abilities (NL), patients with mild cognitive
impairment (MCI), and Alzheimer’s disease (AD)
2 METHOD
2.1 Participants
Fifty Japanese subjects (18 males and 32 females be-
tween the ages of 64 and 92 years) participated in this
study. Table 1 shows the breakdown list of partici-
pants. In this study, all participants are clinically con-
ditioned that CDR of a participant in MCI group and
AD group corresponds to 0.5 and 1, respectively.
2.2 Cognitive Tests
To measure brain function of an elderly during vari-
ous cognitive tests including HDS-R, we have made
a block designed task shown in Fig. 1, and then con-
ducted simultaneous voice-fNIRS measurement dur-
ing cognitive tests. Firstly a participant talks about
the topics of hometown and childhood and answers
for an HDS-R questionnaire test for ten minutes. And
then, he/she does three reminiscence tasks (1. listen-
ing, 2. talking, 3. watching) and three working mem-
ory tasks (1. category recall, 2. reading span, 3. face
recall) for twelve minutes. These six tasks are done
for 60 seconds after rest gazing at a single point on
the display for 60 seconds interval.
2.3 fNIRS Measurement
Functional near-infrared spectroscopy (fNIRS) can
measure neural activity of the cerebral cortex using
infrared rays that are safe to living organisms (Vill-
ringer and Chance, 1997). fNIRS monitors regional
relative changes of oxy/deoxygenated hemoglobin
concentration to measure cortical activation utiliz-
ing the tight coupling between neural activity and
regional cerebral blood flow (Villringer and Firnafl,
1995). This measurement method requires only com-
pact experimental systems and can eliminate physical
restraint from a subject by non-invasive procedures
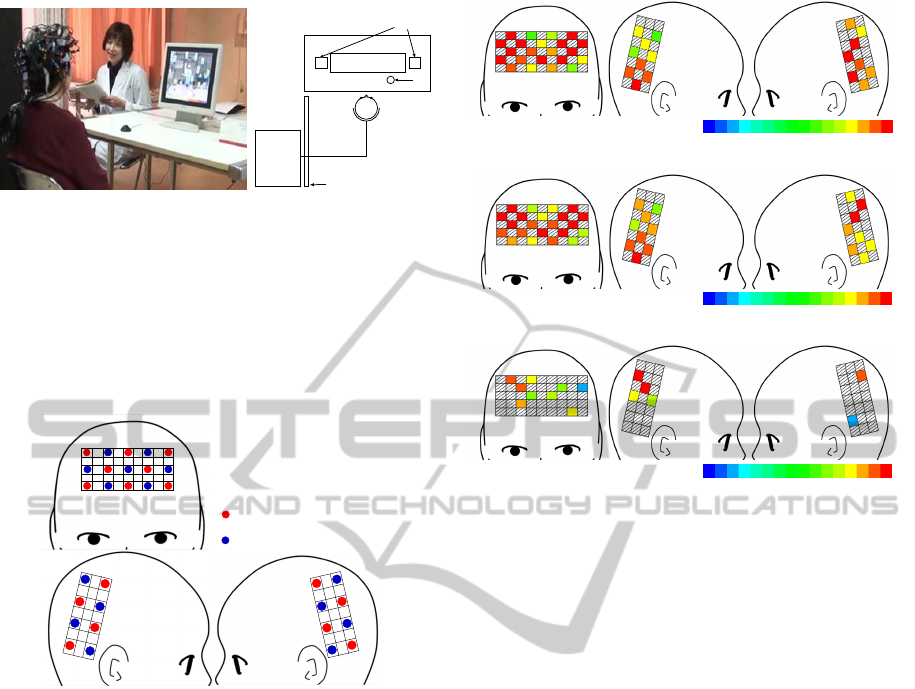
(Fig. 2).
We used the fNIRS topography system FOIRE-
3000 Near-Infrared Brain Function Imaging System
BAYESIAN-BASED EARLY DETECTION OF COGNITIVE IMPAIRMENT IN ELDERLY USING fNIRS SIGNALS
DURING COGNITIVE TESTS
119
fNIRS
indicator
Display
speaker
mic
partition
seated position
Figure 2: Snapshot of fNIRS measurement of an elderly
participant having a cognitive test.
(Shimadzu, Kyoto, Japan), which uses near-infrared
light with wavelengths of 780, 805, and 830 nm. We
set 16 illuminators and 15 detectors in lattice pattern
to form 42 channels (CHs) (22 CHs on frontal lobe,
10 CHs on right parietal and temporal lobe, 10 CHs
on left parietal and temporal lobe) shown in Fig. 3.
N: channel ID
: illuminator
: detector
1
2
3 4
5 6 7 8 9
10 11 12 13
14 15 16 17 18
19
20 21
22
40
37
34
36
38
42
35
39
33
41
26
30
23
27
31
24
28
32
25
29
Figure 3: Channel arrangement of fNIRS measurement.
2.4 Statistical Tests of fNIRS Signals
Preliminary to development of the screening tool, we
have conduct statistical tests of between-group signif-
icant differences using fNIRS signals of oxy-Hb dur-
ing working memory task (1. category recall). We
used two-tailed t-test with significance level of (P<
0.001) after applying Bonferroni’s adjustment (1/42).
Fig. 4 shows the results of t-test for significant dif-
ferences in channel-wise fNIRS signals between any
single pair from NL, MCI, and AD groups. The CHs
that exhibited significant oxy-Hb increase are col-
ored according to the t-values, as shown in the color
bar, while those below the threshold are indicated in
gray. The results indicate the significant difference of
fNIRS signals during cognitive test between normal
group and disease groups. This suggests that fNIRS
signals during cognitive test have potential for detec-
tion of cognitive impairment in elderly patients. Ad-
ditionally, for fNIRs signals during rest, there are no
CHs with significant difference between any single
pair from NL, MCI, and AD groups.
-24
24-12 12
0
t-value
(a) NL group–MCI group
t-value
-30
30-15 15
0
(b) NL group–AD group
-10
10-5 5
0
t-value
(c) MCI group–AD group
Figure 4: Results of t-test for significant differences in
channel-wise fNIRS signals between any single pair from
NL, MCI, and AD groups.
3 CLASSIFICATION OF NL, MCI,
AD GROUPS
The section describes a Bayesian classifier, which
can discriminate among elderly individuals with three
clinical groups: normal cognitive abilities (NL), pa-
tients with mild cognitive impairment (MCI), and
Alzheimer’s disease (AD). To design algorithm for
computer-aided diagnosis of cognitive impairment in
the elderly, we consider the screening process by a
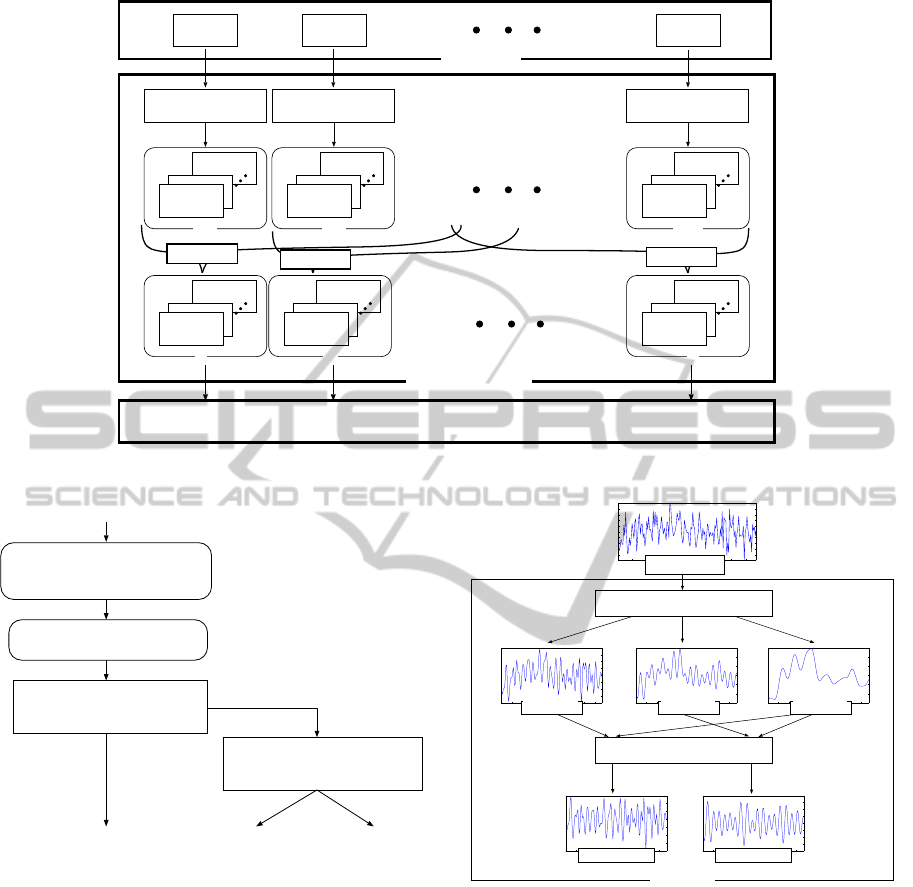
specialist in geriatrics. We thus propose a two-phase
Bayesian classifier shown in Fig. 5 on the assump-
tion of screening process, that firstly checks whether
a suspicion of the cognitive impairment (CI) or not
(NL) from given fNIRS signals; if any, and then sec-
ondly judges the degree of the impairment: MCI or
AD.
3.1 Primitive Analysis of fNIRS Signals
In advance of Bayesian classification, we make a
primitive signal processing fNIRS signals shown in
Fig. 6. Firstly, we make five fNIRS signals every
channels such that noise is reduced by channel-wise
smoothing through three low-pass filters and differ-
ence filters (Fig. 7).
F1 (cutoff freq. 1.92[Hz]): to remove noise arisen from
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
120
CH1
CH2
CH42
Fil2-3
Fil1
Fil2-3
Fil1
Fil2-3
Fil1
CH1
Fil2-3
Fil1
CH2
CH42
Fr
Fil2-3
Fil1
Fil2-3
Fil1
Fc Lr
Original Signal
Primitive Analysis
Feature Extraction
Averaging
Averaging
Averaging
fNIRS fitler
fNIRS fitler
fNIRS fitler
Figure 6: The outline of primitive analysis of fNIRS signals.
fNIRS signals
NB Classifier (1st phase)
NB Classifier (2nd phase)
NL / CI
MCI / AD
NL MCI AD
(42CH oxy-Hb)
cognitive impairment
Feature Extraction
spectoral, statistics 77 features
Primitive Analysis
CH-wise low-path and/or difference filters,
domain averaging
Figure 5: Classification of NL/MCI/AD by two-phase
Bayesian Classifier.
environmental light.
F2 (cutoff freq. 0.96[Hz]): to remove background noise
arisen from biosignal such as pulse wave and blood
pressure.
F3 (cutoff freq. 0.48[Hz]): to remove noise arisen from
body movement such as jaw, eye, neck and so on.
F1-F3: to subtract F3 from F1.
F2-F3: to subtract F3 from F2.
Secondly, we segregate 42 CHs into the following
seven brain areas and then make signal averaging that
integrates fNIRS signals within each of the areas.
Fr: 7 CHs on the right side of frontal lobe (CH:
1,5,6,10,14,15,19).
0 2 4 6 8 10 12 14 16 18
−2
−1.5
−1
−0.5
0
0.5
1
1.5
2
x 10
−3
0 2 4 6 8 10 12 14 16 18
−4
−3
−2
−1
0
1
2
3
x 10
−3
0 2 4 6 8 10 12 14 16 18
−1.5
−1
−0.5
0
0.5
1
1.5
x 10
−3
0 2 4 6 8 10 12 14 16 18
−3
−2
−1
0
1
2
3
x 10
−3
0 2 4 6 8 10 12 14 16 18
−4
−3
−2
−1
0
1
2
3
4
x 10
−3
0 2 4 6 8 10 12 14 16 18
−5
−4
−3
−2
−1
0
1
2
3
4
5
x 10
−3
Original data
Low-pass filter
Filter 2 data Filter 3 data
Filter 1 - 3 data Filter 2 - 3 data
Filter 1 data
0 20
0 20 0 20 0 20
0 20 0 20
(sec)
Difference filter
fNIRS filter
Figure 7: A filter design in fNIRS primitive analysis.
Fc: 8 CHs on the central part of frontal lobe (CH:
2,3,7,11,12,16,20,21).
Fl: 7 CHs on the left side of frontal lobe (CH:
4,8,9,13,17,18,22).
Rf: 5 CHs on the front of right parietal lobe (CH:
23,24,26,27,30).
Rr: 5 CHs on the rear of right temporal lobe (CH:
25,28,29,31,32).
Lf: 5 CHs on the front of left parietal lobe (CH:
33,34,36,37,40).
Lr: 5 CHs on the rear of left temporal lobe (CH:
35,38,39,41,42).
BAYESIAN-BASED EARLY DETECTION OF COGNITIVE IMPAIRMENT IN ELDERLY USING fNIRS SIGNALS
DURING COGNITIVE TESTS
121
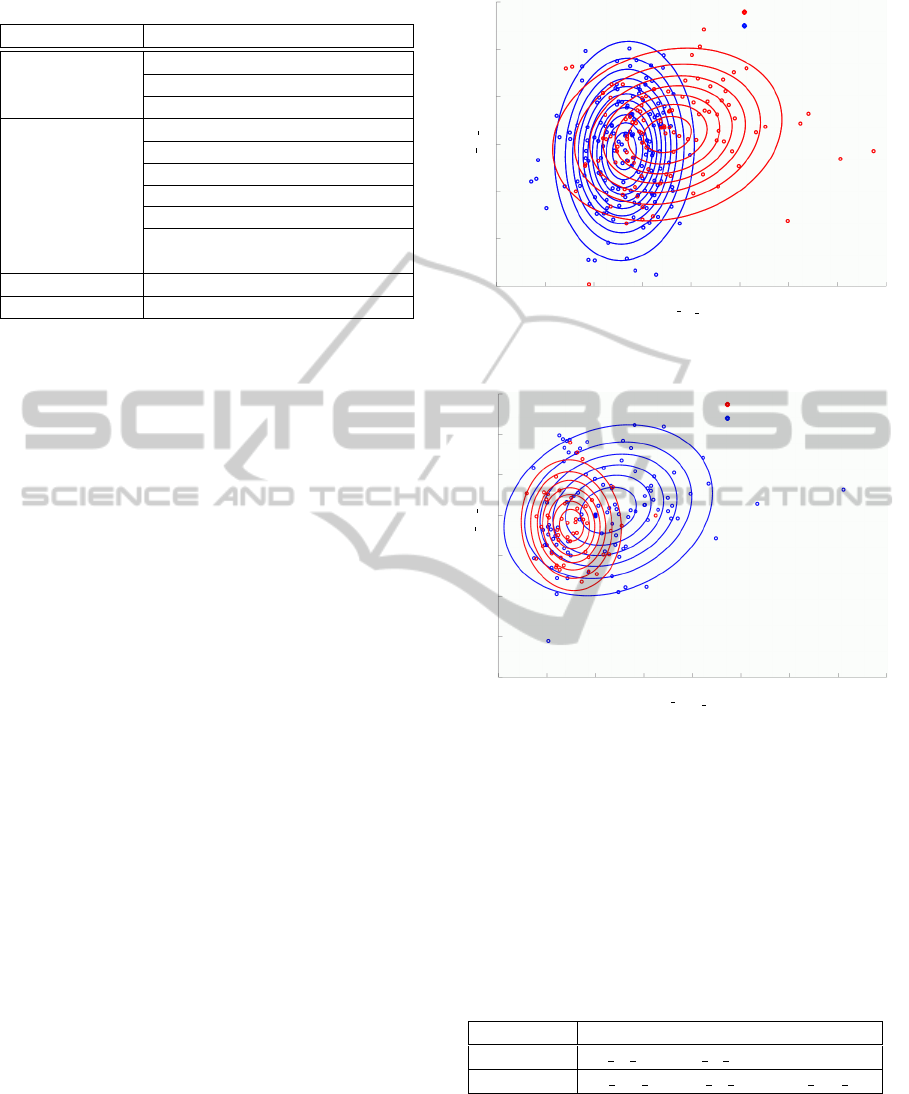
Table 2: fNIRS feature candidates.
fNIRS filtered Feature / Statistics
Filter 1 (F1) Mean value (mean)
Fundamental Frequency (f0)
Centroidal Frequency (fc)
Filter 3 (F3) Maximum value (max)
Minimum value (min)
Variance (var)
Mean value (mean)
Fundamental Frequency (f0)
Gradient of the linear regression
line (gr)
Filter1-3 (F1-3) Variance (var)
Filter2-3 (F2-3) Variance (var)
3.2 Extraction of fNIRS Features
We enumerate features that represents fluctuations of
regional cerebral blood flow if it is the slightest effec-
tive in detection of cognitive impairment, and extract
11 features shown in Table 2 from fNIRS signals in
each of the seven brain areas.
3.3 Bayesian Classifier
In this paper, we adopted naive Bayes classifier
(NB), (Langley et al., 1992) which is a simple
Bayesian classifier with strong independence assump-
tion of attributes (Domingos and Pazzani, 1996). We
construct two classifiers: NB
NL/CI
, which checks
whether a suspicion of the cognitive impairment (CI)
or not (NL) at the first phase, if any suspicion, and
NB
MCI/AD
, which judges the degree of the impair-
ment (MCI or AD) at the second phase.
In our strategy for feature extraction, all of the 77
fNIRS features described above may not be equally
useful and important for discrimination among NL,
MCI, and AD. In this study, we conduct system-
atic feature selection by using the forward stepwise
(FSW) method (Draper and Smith, 1998), which is
the most popular form of feature selection in statistics
and consists of a combination of the forward selection
and backward elimination methods. FSW is a greedy
algorithm that adds the best feature (or deletes the
worst feature) during each round. We make a model
selection method based on the criterion of accuracy
rate of the classification.
4 CLASSIFICATION
ASSESSMENT
We have examined discrimination performance by
-3 -2 -1 0 1 2 3 4 5
-3
-2
-1
0
1
2
3
Lr
F1 fc
Fr
F3 max
Normal cognitive ability
Cognitive Impairment
Figure 8: Distributions of NL/CI estimated by classifier
NB
NL/CI
.
-2 -1 0 1 2 3 4 5 6
-4
-2
-1
0
1
2
3
Lf
F1 mean
Lf
F1−3 var
Alzheimer’s Disease
Mild Cognitive Impairment
-3
Figure 9: Distributions of MCI/AD estimated by classifier
NB
MCI/AD
.
modeling two-phase Bayesian classifiers for discrim-
inating among elderly individuals with NL, MCI, and
AD, by using fNIRS signals of oxy-Hb during work-
ing memory task (1. category recall) (see Fig. 1) col-
lected from 50 participants (see Table 1). Table 3
shows the selected fNIRS features by each of NB
classifiers. To evaluate detection performance, we
adopted leave-one-out cross-validation.
Table 3: Selected fNIRS features.
Classifier Selected Feature
NB
NL/CI
Fr F3 max, Lr F1 fc
NB
MCI/AD
Lf F1-3 var, Lf F1 mean, Fc F1-3 var
Fig. 8 and Fig. 9 show the distributions of NL
group / CI group and MCI group / AD group by clas-
sifiers NB
NL/CI
and NB
MCI/AD
, respectively. In the
figures, 250 samples (145 samples in Fig. 9), such
that fNIRS signals are analyzed after divided into five
spans, are plotted. fNIRS features are mean and vari-
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
122

Table 4: Classification results.
P
P
P
P
P
P
P
clinical
detection
NL MCI AD accuracy
NL 11 7 3 52.4%
MCI 1 14 4 73.7%
AD 0 1 9 90.0%
predictive value 91.7% 63.6% 56.3 68.0%
ance normalize in each variable.
Table 4 shows the confusion matrices and the
statistics of classification results using two-phase
classifiers consist of NB
NL/CI
and NB
MCI/AD
. The re-
sults indicate that both the accuracy rate of AD and
the predictive value of NL are equal to or more than
90%. This means that no subject in AD groups are
misclassified into NL group (only one is misclassified
into MCI group), and that subjects classified into NL
group are not all patient with AD (only one should be
in MCI group). This suggests that proposed approach
is adequate practical to screen the elderly with cogni-
tive impairment. The results that the accuracy rate of
MCI is 73.7% and that most of subjects misclassified
are classified into AD group are both relative accept-
able performance for screening tool.
5 CONCLUSIONS
We developed a new technology for early detec-
tion of cognitive impairment in the elderly, focus-
ing on the brain activity during cognitive task. The
detection method is based on the data mining ap-
proach using Bayesian classification and is simple and
non-invasiveprocedure using functional near-infrared
spectroscopy (fNIRS). We proposed a Bayesian clas-
sifier using fNIRS signals, which can discriminate
among elderly individuals with three clinical groups:
normal cognitive abilities (NL), patients with mild
cognitive impairment (MCI), and Alzheimer’s disease
(AD). This paper also reported the examination of the
detection performance by cross-validation, and the re-
sults that both the accuracy rate of AD and the predic-
tive value of NL are equal to or more than 90%. Con-
sequently, the empirical results suggested that pro-
posed approach is adequate practical to screen the el-
derly with cognitive impairment.
ACKNOWLEDGMENTS
We are grateful to SHIMADZU Corporation, Na-
tional Center for Geriatrics and Gerontology, and If-
com Co. Ltd. for fNIRS measurement system, clin-
ical data collection environment, and data collec-
tion, respectively. This work was supported in part
by SENTAN, Japan Science and Technology Agency
(JST), and part by Adaptable and Seamless Tech-
nology Transfer Program through target-driven R&D,
JST, and part by Suzuken Memorial Foundation.
REFERENCES
Buschke, H., Kuslansky, G., Katz, M., Stewart, W. F., Sli-
winski, M. J., Eckholdt, H. M., and Lipton, R. B.
(1999). Screening for dementia with the Memory Im-
pairment Screen. Neurology, 52(2):231–238.
de Leon, M. J., DeSanti, S., Zinkowski, R., Mehta, P. D.,
Pratico, D., Segal, S., Clark, C., Kerkman, D., De-
Bernardis, J., Li, J., Lair, L., Reisberg, B., Tsui, W.,
and Rusinek, H. (2004). Mri and csf studies in the
early diagnosis of alzheimer’s disease. Journal of In-
ternal Medicine, 256(3):205–223.
de Leon, M. J., Mosconi, L., De Santi, K. B. S., Zinkowski,
R., Mehta, P. D., Pratico, D., Tsui, W., Saint Louis,
L. A., Sobanska, L., Brys, M., Li, Y., Rich, K., Rinne,
J., and Rusinek, H. (2007). Imaging and CSF stud-
ies in the preclinical diagnosis of Alzheimer’s disease.
Annals of New York Academy of Sciences, 1097:114–
145.
Domingos, P. and Pazzani, M. (1996). Beyond Indepen-
dence: Conditions for the Optimality of the Simple
Bayesian Classifier. In Proc. of International Confer-
ence on Machine Learning, pages 105–112.
Draper, N. and Smith, H. (1998). Applied Regression Anal-
ysis (3rd edition). John Wiley & Sons.
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975).
“Mini-Mental State”: A practical method for grading
the cognitive state of patients for the clinician. J. Psy-
chiat. Res, 12(3):189–198.
Imai, Y. and Hasegawa, K. (1994). The revised Hasegawa’s
Dementia Scale (HDS-R): evaluation of its usefulness
as a screening test for dementia. J. Hong Kong Coll.
Psychiatr., 4(SP2):20–24.
Kato, S., Suzuki, Y., Kobayashi, A., Kojima, T., Itoh, H.,
and Homma, A. (2011). Statistical analysis of the
signal and prosodic sign of cognitive impairment in
elderly-speech: a preliminary study. In Proc. of 5th
International Jount Conference on Biomedical Engi-
neering Systems and Technologies (BIOSTEC 2011),
pages 322–327.
Langley, P., Iba, W., and Thompson, K. (1992). An anal-
ysis of Bayesian classifiers. In Proc. of The Tenth
National Conference on Artificial Intelligence (AAAI-
92), pages 223–228.
Morris, J. C. (1993). The Clinical Dementia Rating
(CDR): Current version and scoring rules. Neurology,
43(11):2412–2414.
Mosconi, L., Berti, V., Glodzik, L., Pupi, A., De Santi, S.,
and de Leon, M. J. (2010). Pre-clinical detection of
Alzheimer’s disease using FDG-PET, with or with-
out amyloid imaging. Journal of Alzheimers’ Disease,
20(3):843–854.
BAYESIAN-BASED EARLY DETECTION OF COGNITIVE IMPAIRMENT IN ELDERLY USING fNIRS SIGNALS
DURING COGNITIVE TESTS
123

Villringer, A. and Chance, B. (1997). Non-invasive optical
spectroscopy and imaging of human brain function.
Trends Nurosci., 20:435–442.
Villringer, A. and Firnafl, U. (1995). Coupling of brain
activity and cerebral blood flow: basis of functional
nuroimaging. Cerebrovasc. Brain Metab. Rev., 7:240–
276.
Zhang, D., Wang, Y., Zhou, L., Yuan, H., Shen, D., and the
Alzheimer’s Disease Neuroimaging Initiative (2011).
Multimodal classification of alzheimer’s disease and
mild cognitive impairment. Journal of Neuroimage,
55(3):856–867.
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
124
