
COMPENSATORY MOVEMENT DETECTION
THROUGH INERTIAL SENSOR POSITIONING
FOR POST-STROKE REHABILITATION
Carla M. Borges
1,2
, Claudia Silva
3
, Antonio J. Salazar
1,2
, Ana S. Silva
1,2
, Miguel V. Correia
1,2
,
Rubim S. Santos
3
and João P. Vilas-Boas
4
1
Instituto de Engenharia de Sistemas e Computadores do Porto (INESC Porto), R. Dr. Roberto Frias 378, Porto, Portugal
2
Faculdade de Engenharia, Universidade do Porto, R. Dr. Roberto Frias s/n, Porto, Portugal
3
Centro de Estudos do Movimento e Actividade Humana (ESTSP-IPP), R. Valente Perfeito 322, V. N. Gaia, Portugal
4
CIFI2D, Faculdade de Desporto da Universidade do Porto, Rua Dr. Plácido Costa 91, Porto, Portugal
Keywords: Rehabilitation, Stroke patients, Compensatory movements, Sensor positioning, Accelerometry.
Abstract: An increasing ageing society and consequently rising number of post-stroke related neurological
dysfunction patients are forcing the rehabilitation field to adapt to ever-growing demands. In parallel, an
unprecedented number of research efforts and technological solutions meant for human monitoring are
continuously influencing traditional methodologies, causing paradigm shifts; extending the therapist patient
dynamics. Compensatory movements can be observed in post-stroke patient when performing functional
tasks. Although some controversy remains regarding the functional benefits of compensatory movement as
a way of accomplish a given task, even in the presence of a motor deficit; studies suggest that such
maladaptive strategies may limit the plasticity of the nervous system to enhance neuro-motor recovery. This
preliminary study intends to aid in the development of a system for compensatory movement detection in
stroke patients through the use of accelerometry data. A post-stroke patients group is presented and
discussed, instructed to perform reach and press movements while sensors were positioned at different
location on the arm, forearm and trunk, in order to assess sensor positioning influence. Results suggest that
P1 is advantageous for compensatory elevation movement detection at the shoulder; P4 seems the most
appropriate for detecting the abduction; and P5 presents a reasonable sensitivity for detection of
anteriorization and rotation of the trunk.
1 INTRODUCTION
According to the World Health Organization
(WHO), 15 million people worldwide suffer a stroke
each year, being the leading cause of disability in
adult population (Thrane, Emaus, Askim, Anke,
2011). Stroke is defined as an acute neurological
dysfunction of vascular origin with rapid onset of
signs and symptoms according to the committed
areas of the brain (WHO, 2011). As epidemiological
studies show, disability following stroke can
evidence in the form of neurological dysfunctions
and reduced ability to actively engage in daily
activities, justifying the need for intervention (Geyh
et al., 2004).
Impairment of upper limb function is one of the
most common deficit following stroke, specifically
at the middle cerebral artery (MCA) territory, and to
date, specific rehabilitation remains challenging to a
significant extent, with little agreement on the
procedures to be followed, despite ongoing
published guidelines containing recommendations
on interventions and assessment strategies targeted
towards the diverse areas of post-stroke disability
(Lucca, 2009; Cirstea, Levin, 2007; Geyh et al., 2004).
The predominantly affected arm may present
muscular weakness; abnormal muscle tone, postural
adjustments, and movement synergies;
biomechanical impairments at joints and/or soft
tissues level; incorrect timing of components within
a movement pattern and loss of interjoint
coordination (Cirstea, Ptito, Levin, 2006). In face of
the before mentioned, it is often identified in post-
stroke patients when attempting to move, as in for
reaching an object, the emergence of compensations
297
Borges C., Silva C., J. Salazar A., Silva A., V. Correia M., S. Santos R. and P. Vilas-Boas J..
COMPENSATORY MOVEMENT DETECTION THROUGH INERTIAL SENSOR POSITIONING FOR POST-STROKE REHABILITATION.
DOI: 10.5220/0003798102970302
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2012), pages 297-302
ISBN: 978-989-8425-89-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
related to the available motor strategies and
expressed in form of a pathological synergy
(Michaelsen, Dannenbaum, Levin, 2006).
The neurophysiologic explanation highlights the
post-trauma nervous system’s ability to exploit the
motor system’s redundancy by replacing lost motor
patterns elements with new ones to achieve the
desired task (ib.). In fact, it is well known that after a
lesion, the nervous system can be reorganized
producing an adaptive or maladaptive sensoriomotor
behaviour, highlighting thus the importance of the
reorganization through selective afferent input to
optimize internal representation and influence
movement control (Nudo, 2007; Raine, 2009). In
spite of the mentioned, the use of compensations can
also result in secondary complications such as
muscle weakness or contractures due to joint
misalignment and a lack of recovery of isolated joint
movements, as elbow extension, reinforcing the idea
of the maladaptive nature of such novel movement
patterns post injury (Cirstea and Levin, 2007;
Cirstea, et al., 2006).
Recent advances have promoted the development
of wearable/portable solutions for a number of
human monitoring scenarios. In parallel with such
technological advances, new quantified based
human movement models are commencing to
emerge, applicable to neuromotor assessment.
Kinematic models, based on accelerometry and
angle variation, can estimate 3D arm movement and
events such as falls; however, image based analysis
models seem to dominate, influencing
methodologies and protocols to parallel conventional
medical and rehabilitation observational assessment.
2 METHODOLOGY
2.1 Subjects
The sample was composed by two post-stroke
patients receiving physiotherapy care at a
rehabilitation center, part of an umbrella research
project. Participants had to meet the following
inclusion criteria:
1. Confirmatory neuroimaging results of a single,
unilateral stroke in the MCA territory,
sustained at least 3 months prior.
2. Absence of hemispatial neglect.
3. Absence of major visual, perceptual or
cognitive deficits, confirmed by the mini-
mental state examination (MMSE).
4. Active range of motion in the compromised
arm of at least 15º in the shoulder and elbow.
Explicit exclusion criteria included cerebellar or
brain stem lesions; and pain/sub-luxation in the
upper-limb.
Arm motor impairment was evaluated prior to
measurements, as seen on
Table 1, with the arm
subsection of the Fugl-Meyer scale - FMA (Fugl-
Meyer et al., 1975) and the Reach Performance
Scale - RPS (close target). This clinical evaluation
was performed by a team of three experienced
physiotherapists with more than 10 years of clinical
practice in neurological field.
Table 1: Demographic data and clinical scores of stroke
patients.
Subjects
Patient A Patient B
Age/Gender 49/Male 47/Female
Location of lesion LMCA RMCA
Months post-stroke 66 20
RPS Score (close
target)
5/18 12/18
FMA (shoulder, elbow,
forearm)
4/36 20/36
FMA (wrist) 0/10 2/10
FMA (hand) 2/14 12/14
FMA (coordination) 0/6 3/6
LMCA – Left MCA; RMCA – Right MCA
2.2 Experiment Protocol
The subjects were following, at the time,
conventional rehabilitation procedures associated
with their condition, based on the Bobath Concept
principles. This is a problem-solving approach to the
assessment and treatment of individuals with
disturbances of function, movement and postural
control due to a lesion of the central nervous system
(Raine, 2009). Although sitting balance was not
measured directly, all subjects were ambulatory
without aids and had no difficulty in maintaining a
stable sitting posture during data collection.
As reaching is the most common upper-limb
human gesture, one can understand the great amount
of interest devoted to its analysis, having some
studies reported the expected components of
movement, when target is placed in middle line and
in healthy population: elbow flexion at the beginning
of sequence, followed by combined shoulder
flexion, shoulder horizontal adduction and elbow
extension during the middle and later phases of the
reach (Levin et al., 2004).
Each subject was assessed in sitting position,
with a table placed in front of them, at a height
corresponding to the alignment of the iliac crests.
The table limit was coincident with the distal border
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
298

of the subject’s thigh, so as not to interfere with the
arm trajectory. The subjects were instructed to reach
and press a target placed ipsilaterally to the upper
limb in study, in groups of three repetitions (as to
avoid variations due to fatigue) separated by one
minute rest period.
The target’s placement reference was the
anatomical reaching distance of the hand, using the
measured distance from the acromion to the
metacarpophalangeal joint of the thumb (Reisman
and Scholz, 2006; Vandenberghe, Levin, De
Schutter, Swinnen, Jonkers, 2010). The individual
was instructed, after verbal command, to perform the
functional task. The starting position for the
movement followed: shoulder approximately 0 ° of
flexion / extension and 0 ° of internal rotation, elbow
at approximately 100º of flexion, forearm in
pronation with the palm of the hand resting on thigh
(Wagner, Lang, Sahrmann, Edwards, Dromerick,
2007; Michaelsen, Luta, Roby-Brami, Levin, 2001).
Performance was video recorded for posterior cross-
reference.
2.3 System Description and Setup
A simple wearable monitoring device, named
W2M2 (Wireless Wearable Modular Monitoring),
was designed and implement for inertial data
capturing. The device was based on commercially
available components that could be assembled in a
fast manner, without extensive knowledge of
electronics; seeking to reduce overdependence on
collaborating engineers. The resulting sensor
modules had dimensions of 5.5 x 3 x 2.5 centimeters
The main rehabilitation objectives were focused
on the patient’s affected upper limb. In order to
insure sensor placement repeatability, precise bone
landmarks were required. After a physiological
study of the target area and experimental trial of
sensor positioning for assured subject upper limb
mobility and comfort, the following positions were
considered:
• P1, placed under the acromion, following the
line that connects the lateral epicondyle and the
acromion;
• P2, placed on the middle point between lateral
epicondyle and the acromion;
• P3, immediately above lateral epicondyle, in
alignment with acromion;
• P4, immediately below the lateral epicondyle,
after elbow articulation;
• P5 is in the trunk on the T12.
It should be mentioned that although only these
positions were considered for the present study the
ease with which the patients adapted to the presence
of the sensor permits to imply its use in numerous
other locations.
3 RESULTS
The accelerometers data is captured at a frequency
of approximately 100 Hz, which is then transmitted
wirelessly. A smoothing procedure follows applying
a simple moving average smoothing strategy in
order to reduce the influence of noise and
oscillations. Additional plus/minus pseudo-envelope
functions were generated through a moving window
standard deviation approach, according to
Equation 1, in order to provide visual indicators of
signal stability.
S
envelope
(t)=S
smooth
(
)
±
S
raw
t+
t
w
2
t-
t
w
2
(1)
where:
S
envelope
= envelope function;
f
WSD
= window mean standard
deviation function.
Data was collected from the two target subjects,
using the W2M2 device, at the established points,
for the reach-press and return functional task. A set
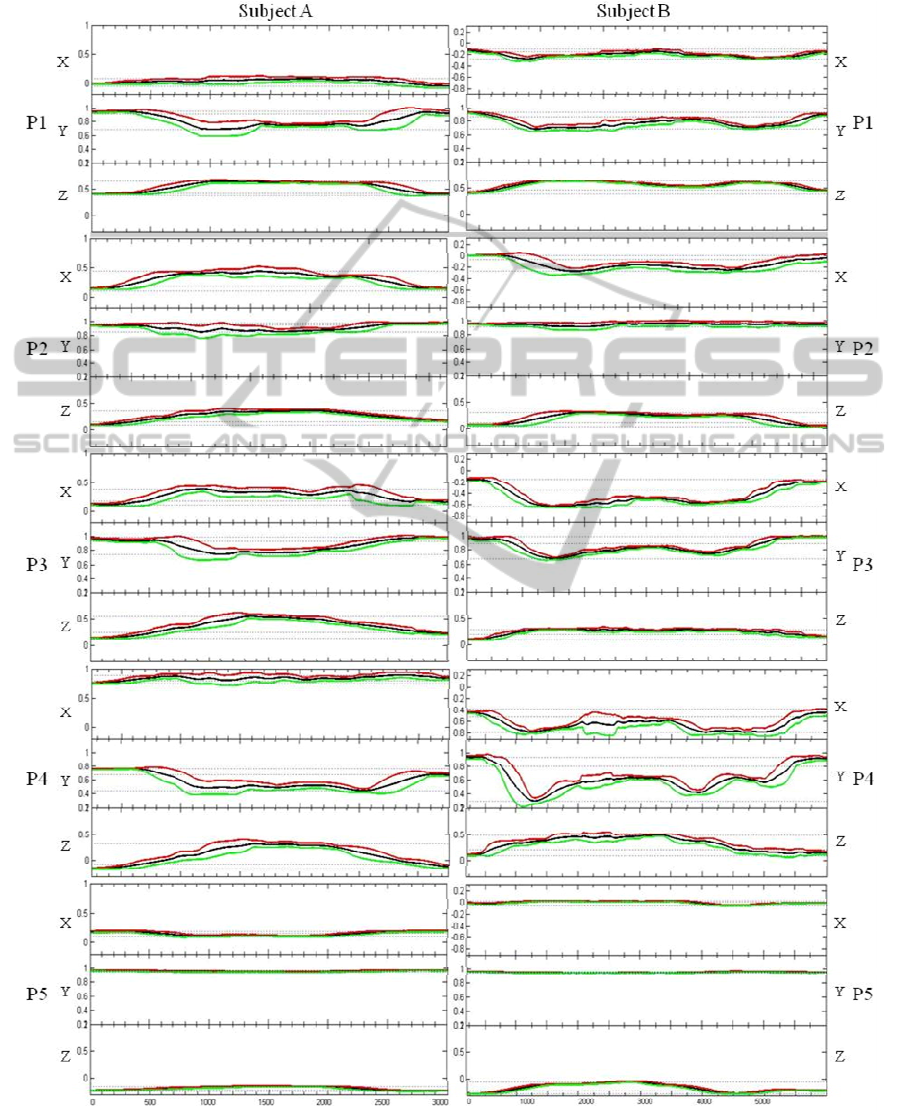
of resulting signals are presented on Figure 1,
accompanied by measurements such as maximum,
minimums, segment amplitude variation and base
calibration references, and corresponding video for
posterior cross-reference. Table 2 shows a
comparative description of movement components,
antero-posterior (A-P), superior-inferior (S-I) and
medial-lateral (M-L), for all sensor locations
analysed. A growing sensitivity scale ranging from 1
to 3 was used for the characterization by a team of
physiotherapists.
Table 2: Sensitivity descriptive analysis of movement
components for sensor locations.
Subject A Subject B
A-P S-I M-L A-P S-I M-L
P1 1 3 1 1 3 2
P2 2 1 1 2 2 2
P3 2 2 2 2 2 2
P4 2 2 3 2 2 3
P5 3 3 3 3 3 3
A-P – Anterior-Posterior; S-I – Superior-Inferior;
M-L – Medial-Lateral
COMPENSATORY MOVEMENT DETECTION THROUGH INERTIAL SENSOR POSITIONING FOR
POST-STROKE REHABILITATION
299

4 DISCUSSION
The sample data is presented in Figure 1 showing
accelerometry data measured at all five sensor
locations (referred to as P1, P2, P3, P4 and P5) for
subjects A and B. The inherent difference in
acceleration amplitudes shown especially in X-axis
between subjects is related to the fact they present
opposite compromise limbs (LMCA vs. RMCA).
The discussion that follows is based on the multiple
data collected from both subjects and their
correspondent video records.
From visual analysis, subject A shows evidence
of reduced segmental selectivity and poor shoulder-
elbow interjoint coordination. Limited motor control
of the upper limb (stability/mobility relation) causes
exaggerated oscillation during movement, which
propagates throughout the body. Compensations on
the movement pattern were visually detected, in
particular excessive elevation and abduction of the
shoulder at the beginning of the movement, as well
as anteriorization and rotation of the right hemi-
trunk at the transport phase. Video analysis
confirmed that the subject did not fully complete the
functional task, i.e., the hand approached but did not
press the target.
Subject B presents increased selectivity in the
movement, observed by the shoulder-elbow
interjoint coordination, and reflected in a reduced
compensatory mechanism through shoulder
abduction. The subject presented a degree of tremor
at the distal segments of the upper limb, evident at
the final phase of the movement, which can be
explained by deficit in the stability/mobility relation.
One also verifies some compensation at the trunk
level, in particular with the anteriorization
component. This individual, comparatively with
subject A, presented increased execution times,
being however important to relate that in contrast
with subject A, has the capacity to fully complete
the task.
In relation with sensor position P1, subject A
presents an average movement in the anterior
direction, i.e. anterior-posterior (X-axis), with
reduced pronunciation (short trajectory), which can
be explained by the incapability of fully reaching the
target. Both patients present on the collected data,
elevation and abduction of the shoulder, at the initial
phase of the movement, corroborating the visual
analysis. Subject B shows that the elevation and
abduction resource is also a strategy used on the
return phase of the movement.
In relation with sensor position P2, there exists
an increased displacement in the anterior direction
(X-axis) when compared with P1; however there is a
lack of marked differences observed on the global
pattern of the movement. Such could suggest that P2
offers more movement detection sensitivity when
compared to P1. In reference to the Y-axis, the
opposite seems to occur, i.e., presents reduced
sensibility for such detection when compared with
P1, for both cases. For Z-axis both individuals do
not present marked differences in the gathered
information from P1 and P2.
Sensor position P3 shows some variability
among the patients. The movement in the anterior
direction (X-axis), performed by subject A is more
pronounced when compared with P1; in turn, for
subject B this movement is better detected when
compared to both P1 and P2. A similar situation
occurs in the remaining movements, i.e. superior
direction (Y-axis) and lateral direction (Z-axis).
Subject B presents no pronounced differences
among the sensor position P1, P2 and P3 for the
lateral direction. This could be explained by lack of
evident movement component recruitment as
compensation during the functional task.
Given the localization of position P4, there exists
a need for redefining the detected movement
components by each of the axis. Thus, the
movement in the antero-posterior direction is now
captured by the Y-axis, and the superior-inferior
direction by the X-axis, remaining the Z-axis
capturing the lateral movements. Subject A, did not
present a significant elevation component (X-axis),
which could be related with the deficit to enlist
selective flexion of the elbow. Subject B presents an
increase elevation component, resulting from an
improved shoulder-elbow interjoint coordination,
being able to perform selective flexion of the elbow
as an integrating part of the movement pattern.
The collected data suggests that sensor position
P1 presents increased commitment between
movement detection in the superior direction
(identification of shoulder elevation as
compensation) and an inter-patient variability;
however a larger number of measurements and
varied sample size is required for such validation.
Finally, as for sensor position P5, one verifies
that such position offers increased reproducibility
among trials, while presenting reduced acceleration
variations (less than 0.1 g in most cases), translating
into a reduced movement of the trunk, especially in
the superior-inferior direction (Y-axis). Some
anteriorization (Z-axis) and rotation (X-axis) is
present, which behave has compensations, given the
reduced capacity of enlisting shoulder flexion with
elbow extension (extensor synergy); implying a
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
300

displacement of the trunk as attempting to reach the
target. Subject B presents increased anteriorization
of the trunk when compared to subject A. The
presence of a larger compensation at this level, in a
clinically less affected individual, could be related to
the difference in functional task completion.
Data analysis seems to suggest that the P1
position is advantageous for compensatory
movement detection at the shoulder level, being
however necessary to complement with information
provided by P5, in order to discriminate between
shoulder or trunk elevation. The information
provided by sensor locations P2 and P3 do not seem
to add relevant knowledge to that provided by sensor
position P1. The P4 position seems the most
appropriate for detecting the abduction component
of the limb; however, in relation with the superior-
inferior movement, this particular sensor position is
insufficient for determination of the corporal
segment where the elevation occurs
(shoulder/elbow/trunk), limiting its reliability for
compensatory movement identification in this
direction. Finally, sensor position P5 presents a good
sensitivity for anteriorization and rotation detection,
though lack of additional comparative data with
other locations at the trunk level.
5 CONCLUSIONS
Methods based on quantitative models can help
therapists and patients to effectively improve the
recovery process, by providing objective assessment
and monitoring, contributing to protocol validation
and information sharing. This preliminary study
focused on the determination of upper limb
associated compensatory movement through
accelerometry data and the influence of sensor
positioning.
ACKNOWLEDGEMENTS
The authors would like to thank the Foundation for
Science and Technology of Portugal for their
support of some of the PhD students involved in this
article (SFRH/BD/61396/2009 and
SFRH/BD/60929/2009). Additionally, the authors
would like to acknowledge the contribution of all
volunteers that took part of the testing procedures.
REFERENCES
Cirstea, M., Levin, M. 2007. Improvement of arm
movement patterns and end-point control depends on
type of feedback in stroke survivors.
Neurorehabilitation Neural Repair, 21, 398-411.
Cirstea, C., Ptito, A., Levin, M. 2006. Feedback and
cognition in arm motor skill reacquisition after stroke.
Stroke. 37, 1237-1242.
Fugl-Meyer, A. R., Jaasko, L., Leyman, I., Olsson, S.,
Steglind, S. 1975. The post-stroke hemiplegic patient.
I. A method for evaluation of physical performance.
Scand J Rehab Med 7, 13-31.
Geyh, S., Cieza, A., Schouten, J., Dickson, H., Frommelt,
P., Omar, Z., Kostanjsek, N., Ring, H., Stucki, G.
2004. ICF core sets for stroke. J Rehabil Med Suppl.
44, 135-141.
Levin, M. F., Desrosiers, J., Beauchemin, D., Bergeron,
N., Rochette, A. 2004. Development and Validation of
a Scale for Rating Motor Compensations Used for
Reaching Patients With Hemiparesis: The Reaching
Performance Scale. Physical Therapy. 84:1, 8-22.
Lucca, L. 2009. Virtual reality and motor rehabilitation of
the upper limb after stroke: a generation of progress? J
Rehabil Med. 41, 1003-1006.
Michaelsen, S.; Dannenbaum, R. and Levin, M. 2006.
Task-specific training with trunk restrain on arm
recovery in stroke – Randomized control trial. Stroke.
37, 186-192.
Michaelsen, S. A. Luta, A., Roby-Brami, A., Levin, M. F.
2001. Effect of trunk restraint on the recovery of
reaching movements in hemiparetic patients. Stroke.
32, 1875-1883.
Nudo, R. 2007. Post-infarct cortical plasticity and
behavioral recovery. Stroke. 38, 840-845.
Raine, S. 2009. The Bobath concept: developments and
current theoretical underpinning. In Raine, Meadows
and Lynch-Ellerington (eds). Bobath Concept –
Theory and clinical practice in neurological
rehabilitation. Wiley-Blackwell.
Reisman, D.S., Scholz, J. P. 2006. Workspace location
influences joint coordination during reaching in post-
stroke hemiparesis. Exp Brain Res. 170, 265-276.
Thrane, G., Emaus, N., Askim, T., Anke, A. 2011. Arm
use in patients with subacute stroke monitored by
accelerometry: association with motor impairment and
influence in self-dependence. J Rehabil Med. 43, 299-
304.
Vandenberghe, A., Levin, O., De Schutter, D., Swinnen, S.
Jonkers, I. 2010. Three-dimensional reaching tasks: effect
of reaching height and width on upper limb kinematics
and muscle activity. Gait & Posture. 32(4), 500-7.
Wagner, J. M., Lang, C. E., Sahrmann, S. A. Edwards, D.,
Dromerick, A. 2007. Sensorimotor impairments and
reaching performance in subjects with poststroke
hemiparesis during the first few months of recovery.
Physical Therapy. 87, 751-765.
World Health Organization. 2011. Stroke,
Cerebrovascular accident. Retrieved from www.who.
int/ topics/cerebrovascular_accident/en/
COMPENSATORY MOVEMENT DETECTION THROUGH INERTIAL SENSOR POSITIONING FOR
POST-STROKE REHABILITATION
301
APPENDIX
Figure 1: Accelerometry data for Subject A and B for locations P1, P2, P3, P4 and P5.
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
302
