
SmarTTransfuser
A Biochip System for the Final ABO Compatibility Test
Karine Charrière
1
, Jean Sebastien Guerrini-Chappuis
1
, Bruno Wacogne
1,2
, Alain Rouleau
2
,
Celine Elie-Caille
2
, Christian Pieralli
2
, Lionel Pazart
1
, Pascal Morel
3
and Wilfrid Boireau
2
1
INSERM-CIT 808, Besançon University Hospital, Besançon, France
2
Institute FEMTO-ST, University of Franche-Comté, Besançon, France
3
French Blood Transfusion Center, Besançon, France
Keywords: Blood transfusion, Lab-on-chip, Microarray, Nanobiotechnology, Surface functionalization, Biosensor,
Bedside test, ABO compatibility, Crossmatch.
Abstract: Before each transfusion of red blood cell concentrate, a final ABO compatibility test is carried out at the
patient's bedside on a piece of card and interpreted visually. Despite this ultimate test, transfusion accidents
still occur due to group incompatibility, which can be lethal. In order to improve this test, we have
developed a specific device based on microarrays for the validation of a smart and safe transfuser in the
context of critical transfusional situations. This miniaturized device incorporates a biochip to analyze ABO
compatibility in order that the hemagglutination reaction of red blood cells with IgMs in solution be
replaced by specific capture and concentration of IgMs on microarrays. Results indicate that a specific
immunocapture is obtained with globular concentrates and with different total blood. Smarttransfuser is a
smart device developed in collaboration with the French Blood Transfusion Center for the optimization at
the patient’s bedside of an ultimate test prior to transfusion.
1 CONTEXT
A variety of blood components are available
including red cell concentrate (RCC), platelet
concentrate (PC) and plasma.
About 24 million components were transfused
and about 15 million whole blood or RCC were
transfused in the USA in 2008 (National Blood
Collection and Utilization Survey 2009). In France,
almost 3 million labile blood products, of which
79% are RCC, are distributed annually for just over
500.000 patients who are transfused.
In 2009 there were 3 patient deaths following a
reaction to ABO-incompatible blood in France, and
this reaction may have contributed to the deaths.
Last year, there were two major ABO-incompatible
reactions, one of which led to death (Afssaps, 2010).
In the UK, a total of 14 ABO-incompatible red cell
transfusions were given, 10 resulting from bedside
administration errors, 2 from wrong blood in tube
phlebotomy errors and 2 due to laboratory errors in
which the wrong sample was used for crossmatch
(SHOT, 2009). According to the Fatalities Reported
to the FDA following blood collection, in combined
fiscal years 2005 through 2010, ABO incompatibili-
ties account for 9% of the transfusion-related
fatalities reported. There was a decrease in ABO
haemolytic reactions from 4 in FY2009 to 2 in
FY2010 (FDA, 2010).
Attentive analysis of these attribution errors has
shown that these mistakes are often multiple,
occurring throughout the transfusional process.
2 ULTIMATE CONTROL
Before each transfusion of RCC in France, a final
ABO compatibility test is carried out at the patient's
bedside on a piece of card and interpreted visually.
The final test, carried out by nurses or doctors, aims
to verify the identity of the recipient (Figure 1). It
also aims to verify compatibility between ABO
grouping and the red blood cell units to be
transfused. This final compatibility test at the
patient’s bedside, the last security measure, is
frequently called into question and other countries
257
Charrière K., Sebastien Guerrini-Chappuis J., Wacogne B., Rouleau A., Elie-Caille C., Pieralli C., Pazart L., Morel P. and Boireau W..
SmarTTransfuser - A Biochip System for the Final ABO Compatibility Test.
DOI: 10.5220/0003852402570262
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 257-262
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
choose other identification methods before
administering red blood cells.
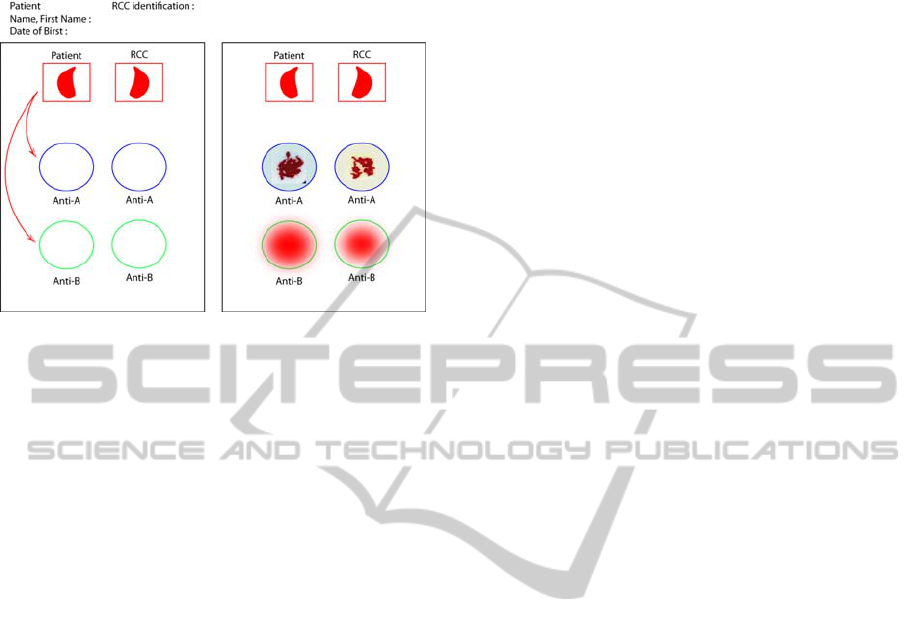
Figure 1: Representation of a bedside card. To use these
cards, it is necessary to rehydrate each reaction field with
one drop of isotonic saline solution (blue and red circles).
One drop of patient blood should be deposited on the
“patient field” and one drop of RCC on the “RCC field”.
Each drop should be deposited in the correspondents’
reaction fields, with a new spatula for each to avoid cross
reaction. The card should be shaken to see whether a
hemagglutination reaction occurs. Nurses should decide
whether RCC can be transfused according to this rule: for
circles of the same color (same antibody), a positive
reaction with blood cells and a negative reaction with the
patient’s blood forbid transfusions. In all other cases,
transfusion is allowed. In this example, transfusion is
allowed.
To eliminate these difficulties, the most
frequently studied solutions aim to ensure
compatibility between the information on the blood
product to be transfused and the recipient. The
technology developed consists of reproduced code
systems: bar codes which are read and radio
frequencies, to verify that the code attributed to the
patient (on a bracelet or in his/her file), corresponds
to the code attributed to the RCC (on a label) in
terms of ABO bulking (Aandahl et al., 2007). These
techniques constitute a higher level of transfusional
safety but nonetheless do not overcome human error,
for example when the bracelet is allocated or during
recipient grouping, etc. (Dzik, 2005). Dzick outlines
in a review some other obstacles: resistance to
change, confusion as to the best technology and
uncertainty concerning investment returns (Dzik,
2007).
In the UK, for example, the final administration
check must be performed at the patient’s side
immediately before administering the blood
component by matching the patient details attached
to the blood component with the details on the
patient’s identification band (or equivalent) (British
Committee for Standards in Haematology 2009).
None of these is entirely satisfactory at the
present time (Levy, 2008), and the frequency of
accidents is noticeably identical, no matter which
test device is used.
The studies described in this position paper aim
to demonstrate the benefit of applying
immunocapture techniques on microarrays to the
development of an “intelligent” blood transfusion
device.
3 IMMUNOSENSOR
ENGINEERING
A great challenge in biosensors and diagnostic
devices is “how to obtain relevant biological
mechanisms on the surface of microarrays and
which analytical tools are convenient for providing
accurate and rapid information on the structures of
captured biological entities of interest?”
To fulfill these aims, many skills must be
combined for a general approach. Successful
immobilization of biomolecules on a solid base
requires several critical factors to be controlled. The
biomolecule must be linked to the surface with
appropriate orientation, false positive signals must
be avoided by minimizing non-specific interactions
and ligands must remain active after binding (no
denaturation, folding, etc.). To control the chemical
functionalization of chips and the self-assembly
processes of chemical monolayers for a highly
controlled surface, biophysical investigations are
needed.
3.1 Design and Prodution of
Homemade Chips
Design and production of homemade chips
compatible with Surface resonance plasmons (SPR)
(from BiacoreTM) have been performed as
previously described with the help of the
MIMENTO technological platform, Besançon,
France (Boireau et al., 2009).
A 2-nm thick chromium (Cr) layer was deposited
on a SiO
2
wafer (width: 13 mm; thickness: 0.17 mm
from AGAR) with plasma sputtering technology to
optimize the adherence of gold to the substrate. The
40 nm thick Au layer was deposited onto the top of
the Cr layer using plasma-sputtering technology.
The deposition time and the argon flow pressure
were optimized to obtain a suitable gold surface. The
deposition time for the Cr and Au layers were
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
258
respectively 5 and 21 s. For all depositions, the
argon flow pressure and current were 7 μbar and
0.3 A respectively.
With these deposition parameters, highly
efficient SPR biochips were produced (in terms of
complex optical thickness, surface roughness and
pseudo-periodic nano-structuration) as demonstrated
by Mangeat et al (Mangeat, 2009).
3.2 Chemical Functionalization and
SPR Experiments
Protein immobilization is a crucial point which
conditions the properties of specificity, stability and
usability of biosensors. Most of the macromolecular
coupling strategies on biosensor surface layers are
based on deposition, functionalization and activation
of polymer cushions with an expected high density
of probes. We chose to control the immobilization
and the homogeneity of the antibody layer on a
bidimensional surface. This was done by SPR
biophysical experiments in order to obtain
quantitative information (level of immobilization).
The homemade chips were chemically
functionalized as follows. The chemical
functionalization was obtained using a mixture of
11-mercapto-1-undecanol (11-MUOH) and 16-
mercapto-1-hexadecanoic acid (16-MHA)
(purchased from Sigma–Aldrich). The mixture of
11-MUOH/16-MHA (97/3 by mole) at 1mM in
absolute ethanol was sonicated for 10 min using an
Elma sonicator (power 90W, frequency 50/60 Hz).
Surfaces were rinsed by ethanol and ultra-pure
water. Then, the carboxyl groups were activated
using 240 µL of N-hydroxysuccinimide (NHS) at 10
mM and 1-Ethyl-3-(3-dimethylaminopropyl)
carbodiimide (EDC) at 48 mM (Amine Coupling Kit
from Biacore AB, Uppsala, Sweden) and incubated
for 30 min at RT. Surfaces were rinsed by ultra-pure
water. This procedure prepares the chips for the
immobilization step.
Biacore experiments were performed with the
Biacore™ 2000 apparatus at 25°C at a rate of 2
µL/min. The antibodies used were IgM anti-A or
IgM anti-B (DIAGAST, provided by the French
Blood Transfusion Center, Besançon). The running
buffer was saline phosphate buffer (PBS, 100 mM at
pH7.4 with NaCl 50 mM). The degree of protein
immobilization and the level of interactions in the
Biacore technology apparatus were plotted on a
sensorgram (response unit (RU) versus time (s)).
One thousand RU correspond to a shift in the
resonance angle of 0.1°. Calibration of the apparatus
gives a correlation between the shift in angle and the
surface mass density deposited on the biochip
surface, ranging from 0.1 to 1 ng/mm
2
(Stenberg,
1991). After exposure to the analytical solution, the
chips were removed from the Biacore unit via an
undock procedure with empty flow cell command.
We showed that after this chemical treatment of
the gold surface, it was possible to immobilize anti-
A and anti-B IgMs. First, different immobilization
pH were tested and the optimal pH conditions for
promoting functionalized antibody/surface
interactions were established. For each antibody, the
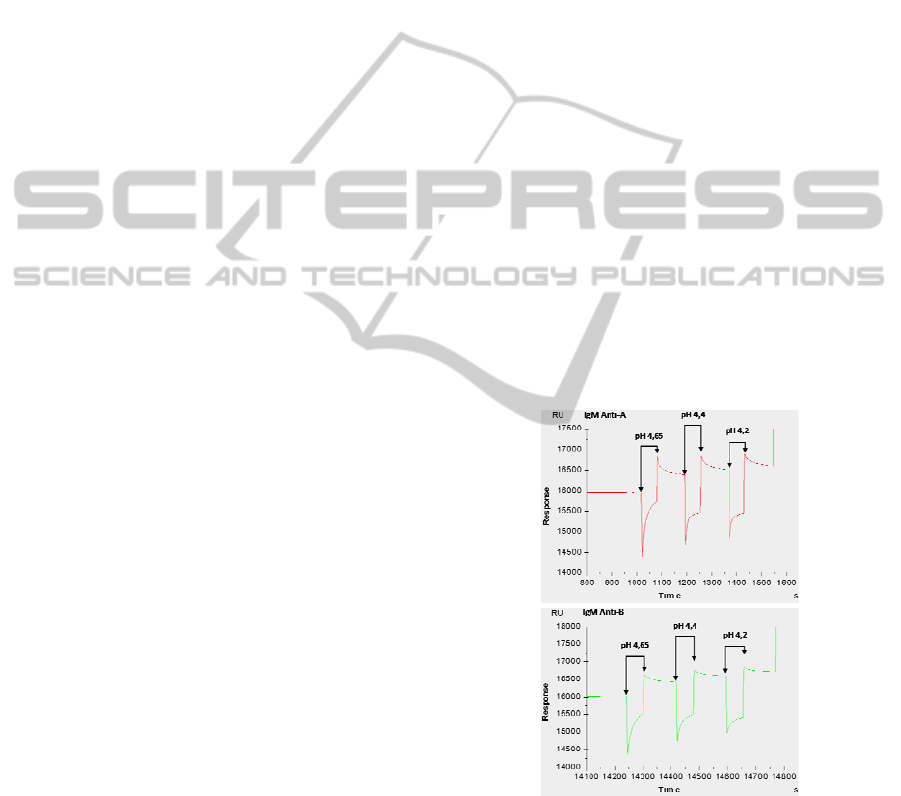
best immobilization was obtained with pH 4.65
(Figure 2). In this way, we were able to implement
efficient conditions for grafting IgM on biochips.
For each antibody, the surface was nearly saturated
after the first injection, showing that our grafting
conditions are optimized (Figure 3). The grafting
rate reaches 1500 IgM/µm
2
on average, which could
potentially involve 100 000 antibodies for each
captured red blood cell.
On these functionalized surfaces, we
demonstrated erythrocyte capture with Atomic Force
Microscopy. With erythrocytes from group A
globular concentrate, we achieved a specific
interaction with surface grafted with anti-A IgMs.
These initial results make way for an ex-vivo
development.
Figure 2: Immobilization of IgM anti-A and IgM anti-B
measured by SPR. Three pHs (4.65; 4.4; 4.2) were tested
and the resonance surface plasmon, expressed in units of
resonance (RU), was measured in real time. For each
antibody, optimum immobilization pH is 4.65.
SmarTTransfuser - A Biochip System for the Final ABO Compatibility Test
259
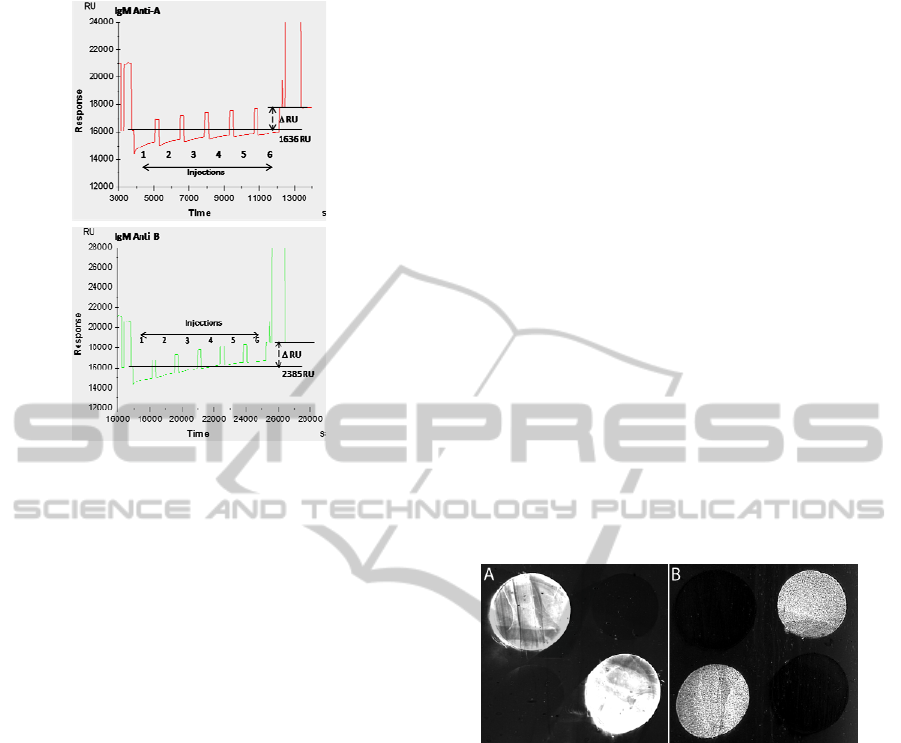
Figure 3: Immobilization of IgM anti-A and IgM anti-B
measured by SPR. Six successive injections were realized
and the resonance surface plasmon, expressed in units of
resonance (RU), was measured in real time. We observed
an echo variation of 1636 RU for the IgM anti-A and of
2385 RU for the IgM anti-B at the end of injections.
3.3 Feasibility Tests
After controlling functionalization of gold surfaces
and immobilization of erythrocytes, we checked the
ability of the biochips to work with other blood
groups. To do this, we conducted surface plasmon
resonance imaging (SPRi) experiments.
The chemical functionalization was performed as
described above. Four spots of IgM antibodies were
grafted onto the surface. Antibodies anti A or anti B
(purchased from Diagast) were diluted (1/10) in
acetate buffer (0.1 mg/mL, pH 4.5) and 2 spots of
each specy (2 µL/spot) were deposited on each
surface and incubated for 1 hour at room
temperature in a humid chamber. Then a blocking
agent (Rat Serum Albumine 40 µg/mL, pH 5.2) was
used to passivate the surface by incubation at room
temperature for 30 min. Incubation in ethanolamine
(0.2 M) was then used to target the free NHS entities
in order to desactivate the surface. Finally, the
biochips were rinsed with ultra pure water and used
for SPRi experiments. They were performed using a
SPRi-Plex imager (Horiba Scientific, France)
equipped with a 660 nm wavelength LED and a
CCD camera.
Experiments were carried out at room
temperature, in physiological serum (NaCl 0.9 %).
The flow rate in the chamber was 50 µl/min.
Ligands (red blood cell concentrate group A or
whole blood groups O, A, AB and B) were injected
(volume 200 µl) and the biochip surface was rinsed
to remove unbound ligands. Whole blood and red
blood cell concentrate were provided by the French
Blood Transfusion Center, Besançon).
The biochip was tested with different blood
groups in order to assess its specificity. Between two
measurements, the surface of the biosensor was
treated with PBS-n-Octyl-beta-D-glucopyranoside
for 1 min to dissociate the probe/target adducts
previously formed (regeneration of the surface
before a second injection). The system has proven to
be selective. Indeed, each target was bound only to
its corresponding antibody. No significant signal
was observed on the non-corresponding antibody
and reference spots. This shows the absence of
undesired non-specific binding (i.e., cross reactivity)
and/or adsorption on the surface. As seen in figure 4,
a very intense signal is observed on anti-A IgM
spots with whole blood group A (Figure 4A)
whereas no significant signal is observed on the two
other spots. In figure 4B, erythrocytes are
immunocaptured only by anti-B IgMs.
Figure 4: Different photographs of one biochip after
injection of whole blood group A (A) or whole blood
group B (B).
This high selectivity demonstrates that it is
possible to determine whole blood / red blood cell
concentrate groups by using a biochip in order to
replace the hemagglutination reaction of red blood
cells by IgM in solution.
4 TOWARD A MEDICAL DEVICE
The sensor should now be incorporated in the main
device, called “SmarTTransfuser” and developed for
clinical trials.
We have previously demonstrated that our
homemade biochips can specifically immunocapture
red blood cells of different blood groups. The next
step of the development of the SmarTTransfuser is
to design a medical device which could
automatically perform the compatibility test at the
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
260

patient's bedside. Indeed, a mobile device is required
which contains the following elements: a fluidic
system, four biochips and an optical reading module.
4.1 General Concept
Our SmarTTransfuser system consists of a secure
patient blood sampling system combined with a
biochip and a mobile reader used to perform an
ultimate blood compatibility test at the patient’s
bedside (Pazart, 2010).
Immunocapture of red blood cells (patient and
RCC) is performed on 4 biochips (2 for the patient
and 2 for the RCC). These biochips are inserted into
a cartridge that includes fluidics arrangements used
to drive the patient’s blood and RCC towards the
chip surfaces. The cartridge is inserted into a mobile
reader/actuator that controls the flow of fluids, the
optical reading of the immunocapture reaction and
allows (or forbids) blood transfusion (Pazart et al.,
2011) (Figure 5).
Figure 5: General concept of the SmarTTransfuser. The
SmarTTransfuser is on the transfusional line, and the
compatibility test is automatically realized (A). If the test
is correct, a green light comes on, the safety measure is
switched off and medical staff continue with the
transfusion as usual (B).
4.2 Immunosensor
The microsystem presented here and developed
under the coordination of the FEMTO-ST Institute is
an embedded detection device, which uses a
microsystem, including the surfaces, for detection of
blood group (biochips).
In order to design the medical device, the first
step was to determine which configuration will
allow for a specific immunocapture. Several
prototypes were designed. The best results were
obtained with the prototype presented in figure 6.
This configuration allows for a good blood flow,
with very little retention of red blood cells.
Figure 6: Representation of the reading zone of the
SmarTTransfuseur system. There are four biochips for the
ABO compatibility test: two for patient blood and two for
red blood cell concentrate (1). To ensure the system was
sealed with no retention of red blood cells, there is a two
part joint: a silicone sheet with a hexagonal window (2)
and a base in polycarbonate (3). All these parts are
inserted in a plastic base (4) and fluids can flow via part 5.
We tested immunocapture with this
configuration. The experimental design was as
follows: NaCl 0.9 % was used as course buffer (50
µL/min) and 250 µL of red blood cell group A
(dilution 1/4) was used (20 µL/min). We showed
that in these conditions, immunocapture is strong
and specific (Figure 7). Optical detection of the red
blood cells has been validated once and the result is
yet to be confirmed. We will therefore not describe
this issue in further detail in this position paper.
Figure 7: Photography of two biochips after
immunocapture reactions with red blood cell concentrate.
Very few red cells are observed on biochip graft with anti-
B antibodies (A and enlargement B). Almost all of the
surface of the biochip grafted with anti-A antibodies is
covered with red blood cells (C and enlargement D).
Detection of the red blood cell capture relies on
optical absorption. Blue LEDs and optical detectors
are positioned on each side of each reading zone.
Optical elements are parts of the mobile
reader/actuator. Optical detection of the red blood
cell trapping has been validated once and the result
SmarTTransfuser - A Biochip System for the Final ABO Compatibility Test
261

has yet to be confirmed. It is therefore too early to
describe this issue in further detail in this position
paper.
5 CONCLUSIONS
The ABO compatibility test is compulsory in
France. Studies led by the national hemovigilance
network show persistent attribution errors of blood
groups leading to transfusional accidents, with the
potential for death in each case (Afssaps, 2004). The
same observations have been made abroad
(Stainsby, 2005).The current procedure for
performing an ultimate pre-transfusional check is
not uniform. Although machines are sent to the
patient’s bedside for an automatic analysis of ABO
grouping, and technology has been developed to
attribute a code to the pouch of blood and to the
patient (Aandahl, 2007), these developments cannot
prevent human errors and transfusional accidents,
which can be lethal.
We have been working on a new system based
on biochips. We have shown through SPRi
techniques that these biochips are very selective and
can replace current hemagglutination test.
With our first tests with the SmarTTransfuseur,
we have shown that immunocapture is specific in
our conditions and that optical detection can be
carried out by absorption measurement. This
medical device could become a new uniform
procedure to carry out the ABO compatibility test.
Because tests will be carried out automatically,
human errors would be avoided and the additional
safety will involve only minor changes to current
practices.
We now need to repeat these tests in order to
cover all transfusional situations before clinical trials
in real-life situations.
The aim of this position paper is not only to
present the system we are developing, but also to
discuss the possibilities and techniques of ultimate
ABO compatibility tests outside France.
ACKNOWLEDGEMENTS
The authors would like to thank the French Blood
Transfusion Center, INSERM, the DGOS, the
CNRS, OSEO and the “innovative project
maturation” programme for their financial support.
REFERENCES
Aandahl, G. S., Knutsen, T. R., Nafstad, K., 2007.
Implementation of ISBT 128, a quality system, a
standardized bar code labeling of blood products
worldwide, electronic transfusion pathway: four years
of experience in Norway. Transfusion, 47(9), p.1674-
1678.
Afssaps, 2010. Rapport annuel hémovigilance. Available
at: http://www.afssaps.fr/.
Afssaps, 2004. Rapport annuel hémovigilance. Available
at: http://www.afssaps.fr/
Boireau, W. et al., 2009. Revisited BIA-MS combination:
entire «on-a-chip» processing leading to the proteins
identification at low femtomole to sub-femtomole
levels. Biosensors & Bioelectronics, 24(5), p.1121-
1127.
British Committee for Standards in Haematology, 2009.
Guideline on the Administration of Blood
Components. Available at: http://www.bcshguidelines.
com/documents/Admin_blood_components_bcsh_050
12010.pdf.
Dzik, W. H., 2007. New technology for transfusion safety.
British Journal of Haematology, 136(2), p.181-190.
Dzik, W. H., 2005. Technology for enhanced transfusion
safety. Hematology / the Education Program of the
American Society of Hematology. American Society
of Hematology. Education Program, p.476-482.
FDA, 2010. Fatalities Reported to FDA Following Blood
Collection and Transfusion. Available at: http://www.
fda.gov/downloads/BiologicsBloodVaccines/SafetyAv
ailability/ReportaProblem/TransfusionDonationFataliti
es/UCM254860.pdf.
Levy, G., 2008. [The pretransfusion bedside agglutination
test is not a «Gold Standard»]. Transfusion Clinique Et
Biologique: Journal De La Société Française De
Transfusion Sanguine, 15(5), p.318-321.
National Blood Collection and Utilization Survey, 2009.
The 2009 national blood collection and utilization
survey report. Available at: http://www.aabb.org/pro
grams/biovigilance/nbcus/Documents/09-nbcus-report.
Pazart L., Wacogne B., Pieralli C., Boireau W., Morel P.,
"Secure perfusion system", Patent WO 2011055031.
Pazart L., Wacogne B., Pieralli C., Boireau W., Morel P.,
"Device for taking a sample of a body fluid and
method for implementing same", Patent WO
2011055029.
SHOT, 2009. Annual Report. Available at: http://www.
shotuk.org/
Stainsby, D., 2005. ABO incompatible transfusions--
experience from the UK Serious Hazards of
Transfusion (SHOT) scheme Transfusions ABO
incompatible. Transfusion Clinique Et Biologique:
Journal De La Société Française De Transfusion
Sanguine, 12(5), p.385-388.
Stenberg, E., 1991. Quantitative determination of surface
concentration of protein with surface plasmon
resonance using radiolabeled proteins. Journal of
Colloid and Interface Science, 143(2), p.513-526.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
262
