
DESIGN OF THE ARTIFICIAL NEURAL NETWORK MODEL
FOR THE PREDICTION OF OUTCOME AFTER STROKE
Jiri Polivka Jr.
1
, Petr Kratochvil
1
, Vladimir Rohan
2
, Jiri Polivka
2
and Jana Kleckova
1
1
Department of Computer Science and Eng., University of West Bohemia, Univerzitni 8, Plzen, Czech Republic
2
Department of Neurology, Charles University Medical School and University Hospital in Plzen
Alej svobody 80, Plzen, Czech Republic
Keywords: Neural network, Stroke, Predictive medicine, Preventive medicine.
Abstract: In our contemporary research we are trying to develop the artificial neural network (ANN) model for the
prediction of outcome after the occurrence of stroke. This paper mentions some important facts about stroke
as well as the urgent need for Computer Assisted Decision Support (CAMS) systems in the relation to
clinical practice. The short review of related studies of ANN in medicine is included. The model input and
output parameters were selected and are also described. The basic ANN design for the predictive model is
mentioned together with the future directions of our research.
1 OVERVIEW
Computer Assisted Decision Support (CADS) in
medicine should enhance the consistency of medical
care in the future. Today there is an expanding range
of medical information stored in electronic form for
each patient, which could be effectively used in
computer-assisted diagnoses systems or preventive
and predictive models. CADS systems are also
excellent tools to cover rare conditions, since no
clinical expert can be expected to possess
encyclopedic knowledge of all of the exceptional
manifestations of diseases.
In our proposed work we tried to set up a new
predictive biomedical model which could be able to
make a prediction of stroke outcomes from the
analysis of various medical input parameters
acquired after patient´s hospitalization. Our
biomedical model uses the Artificial Neural
Networks (ANN) as a new technology for CADS
systems. Neural networks are very universal
instrument of approaching problems. The results
could be used for performing prediction if the output
of the network is continuous or classification if the
outputs are discrete values.
2 STROKE
Stroke is the third leading cause of morbidity and
mortality in the Western world, following ischemic
heart disease and cancer. There are more than 50
million stroke and transient ischemic attack (TIA)
survivors all over the world. More than 1 in 5
survivors may have a subsequent stroke in the next 5
years. The worldwide economic cost of stroke
including direct as well as indirect costs could be
approximately $68.9 billion. Permanent disability
remains a big problem, between 15% and 30% of
stroke survivors suffer permanent disability, 20% of
victims require institutional care within 3 months
after the stroke event (Lloyd-Jones et al., 2009).
One third of stroke patients are under the age of
65 that means a variety of populations are at the risk
and the disease should no longer be considered
confined to the elderly. Women are at a greater risk
for stroke than men. In 2005, women accounted for
60.6% of stroke deaths in the US. The increased
lifespan is the main factor for the increase in stroke
occurrence. However there are many others medical
risk factors including myocardial infarction,
coagulopathies, peripheral vascular disease,
hypertension, atrial fibrillation, or diabetes mellitus.
2.1 Classification of Stroke
The main stroke pathophysiological entites include
thrombosis, embolism, and hemorrhage. Stroke can
be classified as ischemic or hemorrhagic types, with
ischemic stroke accounting for approximately 85%
of the total number. Ischemic stroke occurs due to
467
Polivka Jr. J., Kratochvil P., Rohan V., Polivka J. and Kleckova J..
DESIGN OF THE ARTIFICIAL NEURAL NETWORK MODEL FOR THE PREDICTION OF OUTCOME AFTER STROKE.
DOI: 10.5220/0003875304670470
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2012), pages 467-470
ISBN: 978-989-8425-88-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

either intracranial thrombosis or extracranial
embolism. Intracranial thrombosis is joined to
atherosclerosis, whereas extracranial embolisms
arise from the extracranial arteries or from the
myocardium, often because of concurrent
myocardial infarction, mitral stenosis, endocarditis,
atrial fibrillation or congestive heart failure. The
classification of hemorrhagic stroke can be done as
either intracerebral hemorrhage (ICH) or
subarachnoid hemorrhage (SAH). The common
causes for both ICH and SAH contain hypertension,
trauma, drug use, or vascular malformations (Adams
et al., 1993, Lloyd-Jones et al., 2009). In our case
the TOAST (Trial of Org 10172 in Acute Stroke
Treatment) classification of subtypes of acute
ischemic stroke is used. The acute ischemic stroke
subtypes than include Large-artery atherosclerosis
(embolus/thrombosis), Small-vessel occlusion
(lacune), Stroke of other determined etiology and
Stroke of undetermined etiology. The hemorrhagic
stroke type is also included in the model.
2.2 Clinical Diagnosis of Stroke
Stroke is a medical emergency. The successful
treatment relies especially on its right and well-
timed clinical diagnosis. Great effort in acute stroke
management is focused on correct and rapid
diagnosis and maximal shortening of “onset to
needle time”. It is critical for determining eligibility
for thrombolytic therapy, as the window of
opportunity for therapeutic effectiveness of stroke is
only a few hours (Morgenstern et al., 2004).
There are many new imaging techniques
available which leads to the potential for earlier
opportunities for therapeutic intervention in stroke
patients. The neurological imaging can be used for
the differentiation between hemorrhagic and
ischemic stroke. Important features gained from
brain imaging include detecting early infarction and
determining the location and degrees of infarct and
vascular distribution of the lesions. Computed
tomography (CT) is routinely used in the initial
acute assessment of stroke patient. In acute stroke
case, MRI diffusion-weighted imaging (DWI)
techniques have the ability to differentiate between
various stroke subgroups.
2.3 Therapeutic Intervention in Stroke
The main goal in stroke therapeutic intervention is to
salvage as much cerebral tissue as possible.
Therefore effective thrombolytic therapy must be
initiated rapidly. In 1996, US Food and Drug
Administration (FDA) approved revolutionary
therapeutic intervention with intravenous
recombinant tissue plasminogen activator (rtPA). It
has been used consistently for thrombolysis in acute
stroke. The window of opportunity is less than 4.5 h
from the onset of symptoms (Hacke et al., 2008).
3 ARTIFICIAL NEURAL
NETWORKS IN MEDICINE
The neural networks application in the diagnosis of
cardiovascular disease, primarily in the detection
and classification of at-risk people from their ECG
waveforms was done (Nazeran and Behbehani,
2001). Anoher study uses neural networks to classify
normal and abnormal ECG waveforms and the
abnormal ECG and is described in (Celler and
Chazal, 1998). It made classification of the
waveforms with 70.9% accuracy.
In the next study the ANN which uses non-linear
statistics for pattern recognition was used in
predicting one-year liver disease-related mortality
with the initial clinical evaluation information.The
application of ARTMAP in medicine include
classification of cardiac arrhythmias was described
in (Ham and Han, 1996). The selection of treatment
for schizophrenic and unipolar depressed in-patients
was also made (Modai et al., 1996). Another study
described using of ANN to predict patients with
colorectal cancer more accurately than
clinicopathological methods.
Anothe work based on ANNs is able to detect
ischaemic episodes in long duration ECG recordings
(Papaloukas et al., 2002). The use of the ANN
model as a data mining tool was made to model
complex behaviour of different molecular markers
of dialysis treatment (Elmer et al., 2005). The ANN
model was also used for prediction of
tromboembolic stroke (Shanthi et al., 2010).
4 THE PROPOSED MODEL
The main goal of our proposed system should be the
correct prediction of the stroke outcomes in patients
who were admitted to the hospital with the stroke
diagnosis. The outcome prediction will be made
from the various input medical data processed in the
model. The output describes the overall medical
condition of the stroke patient which is represented
as a grade on some international summarizing scale.
The model outputs (stroke outcomes prediction) are
HEALTHINF 2012 - International Conference on Health Informatics
468
computed for the time points 7 and 90 days after the
stroke occurrence (patient´s admittance to the
hospital).
In the proposed model design we use two
different international scale systems for the stroke.
First is the 42-point National Institutes of Health
Stroke Survey (NIHSS) scale. It was developed to
assist with diagnostic consistency among physicians
and was designed to be completed within 5 to 8 min
(Goldstein and Samsa, 1997). The NIHSS quantifies
neurological deficits in stroke patients. The second
one is The Modified Rankin Scale (mRS). It is a
commonly used scale for measuring the degree of
disability or dependence in the daily activities of
people who had a stroke. This scale is widely used in
clinical outcome measures for stroke clinical trials.
The scale are from 0-6, running from perfect health
without symptoms to death (0 - no symptoms, 1 - no
significant disability, able to carry out all usual
activities, despite some symptoms, 2 - slight
disability, able to look after own affairs without
assistance, but unable to carry out all previous
activities, 3 - moderate disability, requires some
help, but able to walk unassisted, 4 - moderately
severe disability, unable to attend to own bodily
needs without assistance, and unable to walk
unassisted, 5 - severe disability, requires constant
nursing care and attention, bedridden, incontinent, 6
– dead).
4.1 Patient Data and Feature Selection
The various medical inputs for our model were
selected with the help of neurological experts from
the department of neurology, Charles University
Medical School and University Hospital in Plzen.
All stroke patient´s data either for the training set or
the validation set of the model will come from the
University Hospital in Plzen.
The input parameters of the model are listed in
the table 1 (SITS Parameters) and table 2 (non-SITS
parameters). SITS (Safe Implementation of
Treatments in Stroke) is an academic-driven, non-
profit, international collaboration. It is an initiative
by the medical profession to accelerate clinical trials
and to certify excellence in acute and secondary
prevention stroke treatment and to develop
knowledge and leading research. The SITS Network
includes a broad range of hospitals, as well as the
University Hospital in Plzen. The SITS Stroke
Registry is an internet-based interactive stroke
registry developed by SITS. It serves as an
instument for stroke centres to compare own
treatment results with other stroke centres. The basic
parameters which can be found in SITS protocols
were enriched with some other inputs, such as new
laboratory markers of acute stroke or stroke type
classification. This expert´s medical input analysis
and selection of model parameters should ensure
superior predictive accuracy of our biomedical
model.
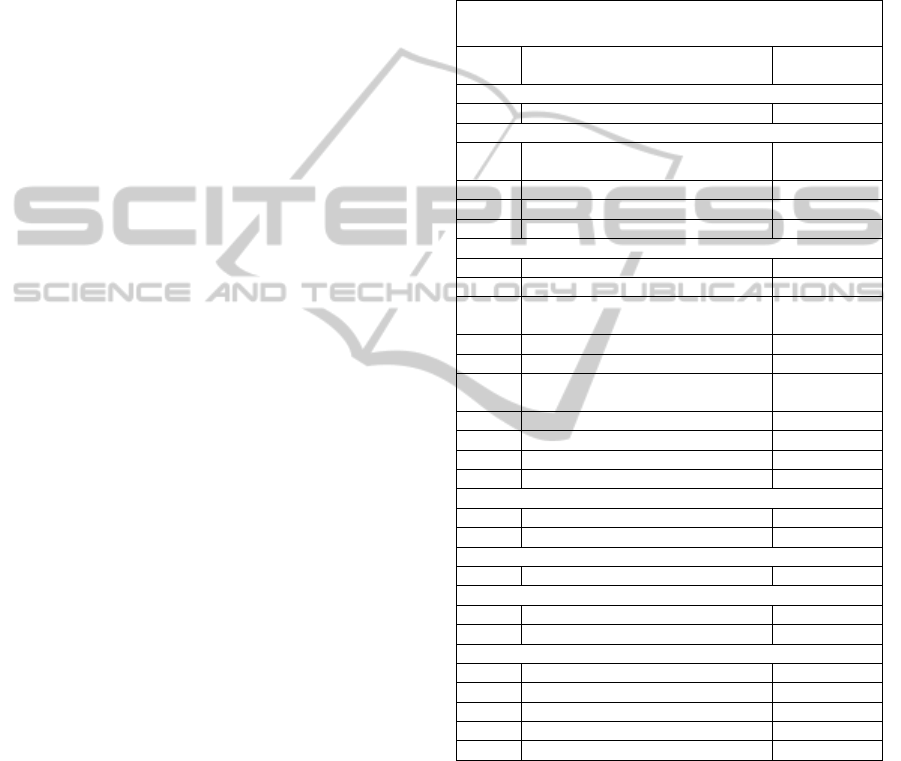
Table 1: SITS input parameters.
SITS Parameters
In.
No.
Input Name Input range
Modified Rankin Scale before stroke
1 mRS score 0 – 6
Prior treatments
2 Antiplatelet tr. (Dypiridamol,
Clopidogrel)
Yes/No
3 Anticolagulants (Heparin) Yes/No
4 Anti – diabetic (Insulin) tr. Yes/No
5 Antihypertensive tr. Yes/No
Risk factors
6 Hypertension Yes/No
7 Diabetes (Dg. of diabetes) Yes/No
8 Hyperlipidemia (Dg.of
hyperlipidemia)
Yes/No
9 Current Smoker Yes/No
10 Previous Smoker Yes/No
11 Previous Stroke (earlier than 3
months)
Yes/No
12 Previous Stroke (within 3 months) Yes/No
13 Previous TIA / Amaurosis fugax Yes/No
14 Atrial fibrillation Yes/No
15 Congestive heart failure Yes/No
Laboratory Indicators
16 Glucose (mmol/l) Number
17 Cholesterol (mmol/l) Number
NIHS
18 NIH Score 0 – 42
Imaging – CT
19 CT current infarct Yes/No
20 Local haemorrhage Yes/No
Other Parameters
21 Age Number
22 Sex M / F
23 Weight Number
24 Systolic blood pressure (mmHg) Number
25 Diastolic blood pressure (mmHg) Number
5 THE ARCHITECTURE OF ANN
The architecture of the artificial neural network is
the multilayered feed-forward network with 37 input
nodes (in the future 41). The first experimental case
uses 20 hidden nodes. The output is designed as one
node which could be able to calculate the overall
patient’s stroke outcome in the international scale of
NIHSS and RANKIN.
DESIGN OF THE ARTIFICIAL NEURAL NETWORK MODEL FOR THE PREDICTION OF OUTCOME AFTER
STROKE
469

Table 2: Non-SITS input parameters.
Non SITS parameters
In. No. Input Name Input
range
Treatment
26 I.V. Trombolysis Yes/No
27 Stroke care unit Yes/No
Stroke Type
28 Large-artery atherosclerosis Yes/No
29 Cardioembolism Yes/No
30 Small-vessel occlusion Yes/No
31 Stroke of other determined
etiology
Yes/No
32 Haemorrhalgic stroke Yes/No
Laboratory indicators
33 CRP Number
34 Platelets Number
35 Leukocytes Number
36 Fibrinogen Number
37 Vitamin D Number
Other possible laboratory indicators (in the future)
38,39,40,41 IL 6, SAA, NSE, PARK 7 Number
6 CONCLUSIONS
In this paper we have discussed a design of our new
biomedical model for the stroke outcome prediction
which is based on artificial neural network
architecture. First important part of the model
creation was the selection of input and output
parameters. This task was done with the help of
neurological experts from the University Hospital in
Plzen. Than the architecture of an ANN was
designed and also briefly referred in this paper. Now
the model is prepared for training patient´s data set.
The ANN training and optimization of the model are
our main research tasks to the future. The work
presented in this paper is supported by The Czech
Science Foundation project 106/09/0740 dealing
with brain perfusion modelling.
REFERENCES
Adams H. P. Jr., Bendixen B. H., Kappelle L. J., Biller J.,
Love B. B., Gordon D. L., Marsh E. E., 3
rd
, 1993.
Classification of subtype of acute ischemic stroke:
definitions for use in a multicenter clinical trial.
TOAST. Trial of Org 10172 in Acute Stroke
Treatment. Stroke 1993;24:35– 41.
Celler, B. G., Chazal, P., 1998. Low computational cost
classifiers for ECG diagnosis using neural networks.
Proceedings of the International Conference of
Engineering in Medicine & Biology Society (EMBC
1998), pp. 1337–1340.
Elmer Andres Fernández, Rodolfo Valtuille, Jesus
Rodriguez Presedo, and Peter Willshaw (2005)
Comparison of Standard and Artificial Neural Network
Estimators of Hemodialysis Adequacy. International
Center for Artificial Organs and Transplantation.
29(2):159–165, Blackwell Publishing, Inc.
Goldstein L. B., Samsa G. P. Reliability of the National
Institutes of Health Stroke Scale: extensit to non-
neurologists in the context of a clinical trial. Stroke
1997;28:307–10.
Hacke W., Kaste M., Bluhmki E., Brozman M., Da´ valos
A., Guidetti D. et al. Thrombolysis with alteplase 3 to
4.5 hours after acute ischemic stroke. N Engl J Med
2008;359:1317–29.
Ham, P. M. and Han, S., "Classification of cardiac
arrhythmias using fuzzy artmap" IEEE Transactions
on Biomedical Engineering, 43(4): 425–430 (1996).
Lloyd-Jones D., Adams R., Carnethon M., De Simone G.,
Ferguson T. B., Flegal K., et al. Heart disease and
stroke statistics—2009 update: a report from the
American Heart Association Statistics Committee and
Stroke Statistics Subcommittee. Circulation
2009;119:e21–181.
Modai, I., Israel, A., Mendel, S., Hines, E.L. and
Weizman, R., "Neural network based on adaptive
resonance theory as compared to experts in suggesting
treatment for schizophrenic and unipolar depressed in
patients," Journal of Medical Systems, 20(6): 403–412
(1996).
Morgenstern L. B., Lisabeth L. D., Mecozzi A. C., Smith
M. A., Longwell P. J., McFarling D. A., Risser J. M.
A population-based study of acute stroke and TIA
diagnosis. Neurology 2004;62:895–900.
Nazeran, H., Behbehani, K., 2001. Neural networks in
processing and analysis of biomedical signals. In M.
Akay (Ed.), Nonlinear biomedical signal processing:
Fuzzy logic, neural networks and new algorithms, pp.
69–97.
Papaloukas, C.,Fotiadis, D. I.,Likas, A., and Michalis, L.
K., 2002. An ischemia detection method based on
artificial neural networks. Artificial Intelligence in
Medicine, 24, 167– 178.
Shanthi, D., Sahoo, G. and Saravanan, N., 2010. Designing
an Artificial Neural Network Model for the Prediction
of Thrombo-embolic Stroke, International Journals of
Biometric and Bioinformatics (IJBB)
, Volume (3)
SITS International, https://sitsinternational.org
HEALTHINF 2012 - International Conference on Health Informatics
470
