
Development of a Self-regenerative Unit for Carbon
Dioxide Removal from Anaesthetic Circuits: Preliminary
Results using Hydroxide Solutions
Inês Pantaleão
1
, Joana Cabral
1
, Joaquim Gabriel
2
, José Sousa
1,3
and Adélio Mendes
1
1
University of Porto, Faculty of Engineering, Chemical Engineering Department, LEPAE,
Rua Dr. Roberto Frias, 4200-465 Porto, Portugal
2
IDMEC-Polo FEUP, University of Porto, Faculty of Engineering,
Rua Dr. Roberto Frias, 4200-465 Porto, Portugal
3
Chemistry Department, University of Trás-os-Montes e Alto Douro,
Apartado 202, 5001-911 Vila-Real Codex, Portugal
Abstract. Carbon dioxide (CO
2
) removal from anaesthetic circuits is currently
performed by soda-lime canisters. However, this procedure has many
drawbacks, being the most important its relation with post operative cognitive
decline and death in the first year after surgery. In this paper an alternative
technology is proposed based on a self-regenerative unit using hollow fiber
membrane contactors and amino acid salts for gas-liquid absorption of CO
2
.
This technology, is expected to overcome all the drawbacks associated with
soda-lime units. A preliminary experiment was evaluated under real conditions
for CO
2
removal, using hydroxide solutions. The system performed similarly to
soda-lime canisters, being able to remove CO
2
from 5 % to 0.5 % (in nitrogen)
during an hour, from a 10 L/min gas stream.
1 Introduction
In anaesthesia, when a low flow circle breathing circuit is used, the patient is
continuously ventilated with a gaseous mixture typically composed by nitrous oxide
(N
2
O), as carrier and anaesthetic gas, oxygen and 1 – 2 % fluorated additives
(sevoflurane/desflurane, being sevoflurane the most frequent). The carbon dioxide
(CO
2
) produced by the patient’s breathing must be removed since the gas flows in a
circle circuit.
Over decades, the use of hydroxide-based absorbents such as soda lime or
Baralyme® was accepted to be a safe and reliable method for removing CO
2
from
anaesthetic gas circuits. However, some evidences were found in recent years
regarding the hazardousness of this approach: bacteria, viruses and spores are
transmitted between patients [8], carbon monoxide can be generated, particularly
when desflourane is used [7] and there is a risk of explosion, since hydrogen is
formed and the temperature in the soda lime canisters is high [4]. The exhausted soda
lime canisters are solid waste which is dangerous and expensive to treat [5].
Moreover, the residual emission of N
2
O and flourinated anaesthetic compounds to the
atmosphere makes this process dangerous for the patient, medical staff and the
Pantaleão I., Cabral J., Gabriel J., Sousa J. and Mendes A..
Development of a Self-regenerative Unit for Carbon Dioxide Removal from Anaesthetic Circuits: Preliminary Results using Hydroxide Solutions -
Preliminary Results using Hydroxide Solutions.
DOI: 10.5220/0003883800690075
In Proceedings of the International Workshop on Veterinary Biosignals and Biodevices (VBB-2012), pages 69-75
ISBN: 978-989-8425-94-2
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
environment. But the worst side effect of the current CO
2
removal system is the
formation of neurotoxic compounds. The high temperatures developed during the
caustic absorption degrades sevoflurane [2], one of the most commonly used
anaesthetic, resulting in the so called compounds A, B, C, D and E [1], referred to as
neuro and nepho-toxic [3]. Their neurotoxicity is associated to the occurrence of post
operative cognitive decline (POCD) which increases exponentially after the age of 65.
Additionally, POCD is related to the occurrence of death in the first year after surgery
(Doherty, 2007).
Our proposal is to develop an alternative CO
2
removal method from anaesthetic
circuits, using hollow fiber membrane contactors and amino acid salts solutions. The
use of hollow fibers provides a large gas-liquid contact area, allowing the
development of a simple and compact unit. Being dense, the membranes block
microorganisms as well as virus transmission. The use of amino acid solutions grants
a biocompatible system and side-reactions of the anaesthetics are eliminated.
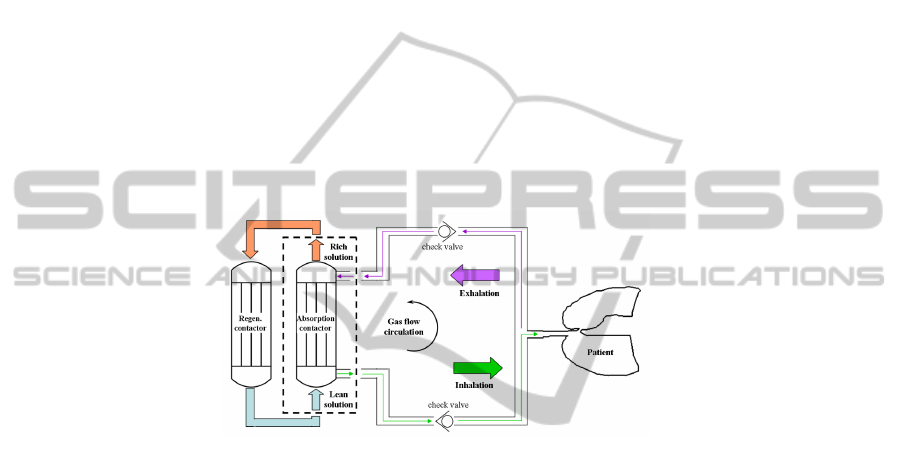
Furthermore, a self-regenerative unit will overcome environmental issues.
The unit will include two contactors (Fig. 1), one to absorb the CO
2
from the
patient’s breathing and the other to regenerate the absorbent solution.
Fig. 1. Sketch of an anaesthetic circuit and self-regenerative unit for CO
2
removal [6].
The absorbent must have a high cyclic absorption yielding, i.e., have a good
absorption rate and a high cyclic absorption capability. It should also be easily
regenerable under acceptable conditions (such as temperature), exhibit very low
volatility and present thermal and chemical stability while simultaneously having a
reasonable price. Since aqueous solutions of salts of most of the natural amino acids
fulfil these requirements, and simultaneously are biocompatible compounds, they
were chosen to be tested as absorbent solutions.
In the present study, the absorption step was studied using the same gas flow rates
as used in anaesthesia, and a commercial cross-flow membrane contactor (Quadrox,
Maquet cardiovascular, Fig. 2). As liquid absorbents, two hydroxide solutions were
tested to validate the concept and the set-up, since there was no need for the absorbent
to be regenerable. Moreover, alkaline solutions are commonly used for acid gas
absorption. The overall performance of the system for CO
2
removal from anaesthetic
gas circuits was evaluated in the laboratory.
70

2 Experimental Set-up
As a preliminary experiment, a commercial membrane module – Quadrox (Maquet),
blood oxygenator – was used with common gas flow rates and aqueous hydroxide
solutions as absorbents. The objective was to study the suitability of this commercial
membrane contactor for CO
2
removal from anaesthetic circuits, by chemical reaction
with a liquid absorbent.
The Quadrox module was adapted to be used in this set-up (Fig. 3). The original
blood circuit was used for the hydroxide solution. The blood outlet was blocked; the
recirculation exit was used instead. The internal heat exchanger shown in Fig. 2 was
not used. The original gas circuit was used for the gas current. The gas outlet was
divided into two tubes: one of them was directed to a CO
2
infrared gas analyzer
(Servomex) and the other to the atmosphere. This was necessary because the CO
2
analyzer used had a limit flow rate of 6 L/min.
The liquid absorbents flowed through the hollow fiber membranes with a flow
rate of 1 l/min. This was achieved using a peristaltic pump (Ismatec). The gas mixture
was made using thermal gas flow controllers (Bronkhorst).
Fig. 2. Flow directions of gas, blood and water (heat exchanger) in the Quadrox module
(retrieved from www.maquet.com).
3 Experiment
The hydroxide solution should be able to remove CO
2
from an anaesthetic gas current
of 10 L/min, with 5 % CO
2
, during a given period of time. The goal was to obtain, at
the exit of the contactor, a gas current with 0.5 % CO
2
.
In a hydroxide solution, CO
2
reacts with the hydroxide ions (HO
-
) according to
equation 1:
2
232
CO ( ) 2HO ( ) CO ( ) H O( )
g
aq aq l
−−
+↔+
(1)
To continuously remove CO
2
from a gas current of 10 L/min with 5 % CO
2
,
during an 8-hour surgery, the amount of HO
-
ions necessary is very high, what could
damage the membranes in the Quadrox module. Therefore, to protect the membranes,
a concentration of 0.2 mol/L was used, which corresponds to a pH of 13.3 (Fig. 4).
71
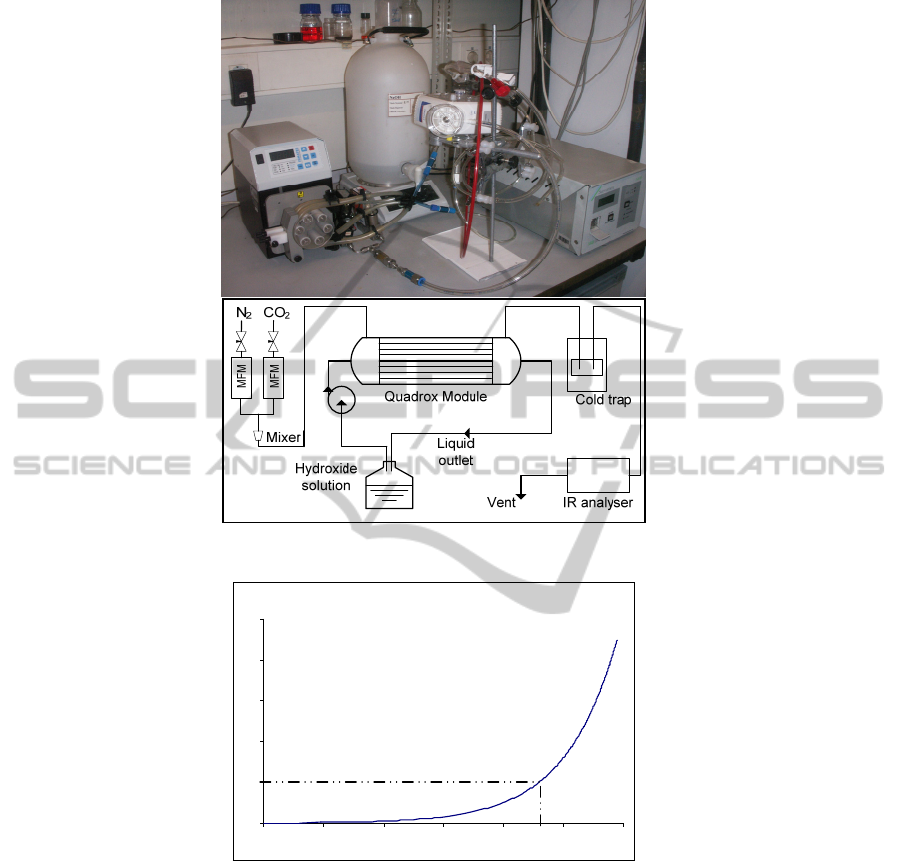
Fig. 3. Picture (a) and sketch (b) of the experimental set-up.
Fig. 4. pH as a function of hydroxide ion concentration [HO
-
], mol/l.
3.1 Test with a Saturated Salt Solution
To maintain the efficiency of the CO
2
removal process, the feeding solution should
have a constant concentration of at least 0.2 mol/L. Therefore, to reduce the volume
of solution necessary, a saturated solution of Barium hydroxide (Ba(HO)
2
) was used
since this salt produces a saturated solution with ca. pH=13. Reacting with CO
2
this
salt produces barium carbonate (BaCO
3
(s)) as a precipitate.
0
0.2
0.4
0.6
0.8
1
11.0 11.5 12.0 12.5 13.0 13.5 14.0
pH
[HO
-
], m ol/l
(b)
HO
-
solution
Peristaltic
pump
CO
2
analyser
Quadrox
module
(a)
72

Since the membrane module used (Quadrox-i) was made of microporous fibers,
precipitation had to be reduced to the minimum possible, since it could block the
membrane pores. Therefore a sodium hydroxide (NaHO) was used to prepare a
solution with concentration 0.215 mol/L, to which solid Ba(HO)
2
was added in order
to maintain the solution saturated. A magnetic stirring was used to keep the solution
homogenized and help the salt to dissolve, maintaining constant the amount of HO
-
ions in solution. Several goals are achieved with this procedure: the efficiency of the
process is maintained constant; HO
-
concentration is kept at the minimum possible;
precipitation is reduced to the minimum possible; the volume of solution necessary is
reduced to the minimum possible.
3.2 Test with Sodium Hydroxide
In this experiment, five litres of NaHO 0.25 M were prepared and used as absorbent
for the CO
2
in the gas current, flowing through the membrane module at 1 L/min. A
higher concentration solution – NaHO 2 M – was added to the liquid container,
whenever necessary, in small amounts of 150 ml, to give back to the solution the HO
-
ions it had already lost by chemical reaction with CO
2
.
4 Results and Discussion
The test with Ba(HO)
2
proved that it is not possible to use microporous fibers
whenever a precipitate is formed, since it blocks the membrane pores. The run lasted
only a couple of minutes, since the precipitate (BaCO
3
(s)) and Ba(HO)
2
itself started
blocking the membrane. The use of saturated solutions produced by insoluble salts
and accompanied by the formation of precipitates is therefore not suitable for CO
2
removal from anaesthetic circuits when using contactors made of porous membranes.
To use this set-up successfully with saturated salts solutions, a dense membrane
module should be considered – Quadrox-D instead of Quadrox-i.
Since the membrane module used was made of porous fibers, sodium hydroxide
(NaHO) was tested, as an alternative to Ba(HO)
2
. Sodium hydroxide is extremely
soluble, easy to handle and cheap. For an inlet gas current of 10 L/min, 5 % CO
2
in
N
2
, the composition of the outlet gas stream was 0.5 % CO
2
for ca. 7 minutes, after
what the CO
2
% started to rise. At this point, ca. 150 ml NaHO solution with
concentration 2 M were added to the plastic container. This highly concentrated
solution was able to give back to the original solution, the HO
-
ions it had already lost
by reaction with CO
2
. The process was repeated whenever necessary (every ca. 7
minutes): when the CO
2
% at the outlet of the module started to rise, the addition of
NaHO was able to maintain the overall efficiency.
This set-up was successfully tested at LEPAE for an hour. During this hour ca. 2
L of NaHO 2 M were added to the original solution in the plastic container. Further
consecutive additions of NaHO 2 M would make the experiment last as long as
necessary, within the life-time of the hollow fiber membranes under these harsh
experimental conditions.
73

This set-up will be ultimately validated by real anaesthesia experiments with
animals, and subsequently with humans.
5 Conclusions
The use of membrane contactors and amino acid salt solutions for CO
2
removal from
gas streams is an innovative approach that presents several advantages over current
absorbent systems used in anaesthetic machines. The technological innovation
introduced by the present work will bring a significant improvement. It will enhance
issues related to the patient’s health and well being, add economical advantages while
simultaneously originating a more environmentally friendly process.
This preliminary experiment with hydroxide solutions to remove CO
2
from
anaesthetic gas streams produced successful results. The set-up has proven capable of
removing CO
2
from 5 % (in a gas stream of 10 L/min) to 0.5 % for an hour. The
experiment could have lasted longer if further additions of NaHO solution were made,
assuming that the limiting factor is the life-time of the membranes, under the given
experimental conditions.
This set-up performs similarly to, and could therefore replace, soda lime canisters
(currently used for CO
2
removal). There is however a drawback which lies in the need
to keep adding NaHO solution to the container. This should no longer be an issue
when amino acid salt solutions are used. In this case, the absorbent solution will
absorb CO
2
and it will afterwards be regenerated in a separate membrane module, in a
cyclic process.
Acknowledgements
The authors wish to thank Maquet for kindly providing the Quadrox module. The
work of Inês Pantaleão and Joana Cabral is supported by the Portuguese Foundation
for Science and Technology (FCT), grant references SFRH/BDE/33802/2009 and
SFRH/BPD/64735/2009, respectively. The research is also supported by funds from
project PTDC/EQU-QUE/114944/2009.
References
1. Cunningham, D. D., Huang, S., Webster, J., Mayoral, J., Grabenkort, R. W., 1996.
Sevoflurane degradation to compound A in anaesthesia breathing systems” British Journal
of Anaesthesia, 77, 537-543.
2. Fang, Z. X. , Kandel, L., Laster, M. J., Ionescu, P., Eger, E. I., 1996. Factors Affecting
Production of Compound A from the Interaction of Sevoflurane with Baralyme® and Soda
Lime. Anesthesia & Analgesia 82, 775-781.
3. Konat, G. W., Kofke, W. A., Miric, S., 2003. Toxicity of Compound A to C6 Rat Glioma
Cells. Metabolic Brain Disease, 18, 11-15.
74

4. Lagorsse, S., Magalhães, F. D., Mendes, A., 2007. Xenon recycling in an anaesthetic
closed-system using carbon molecular sieve membranes. Journal of Membrane Science
301, 29-38.
5. Mendes, A., 2000. Development of an Adsorption/membrane Based System for Carbon
Dioxide, Nitrogen and Spur Gases Removal from a Nitrous Oxide and Xenon Anaesthetic
Closed Loop. Applied Cardiopulmonary Pathophysiology 2, 156-163.
6. Portugal, A. F., Magalhães, F. D., Mendes, A., 2009. Carbon dioxide removal from
anaesthetic gas circuits using hollow fiber membrane contactors with amino acid salt
solutions. Journal of Membrane Science 339, 275-286.
7. Struys, M. M. R. F., Bouche, M. P. L. A., Rolly, G., Vandevivere, Y. D. I., Dyzers, D.,
Goeteyn, W., Versichelen, L. F. M., Van Bocxlaer, J. F. P., Mortier, E. P., 2004. Production
of compound A and carbon monoxide in circle systems: an in vitro comparison of two
carbon dioxide absorbents. Anaesthesia, 59, 584-589.
8. Wilkers, A. R., 2005. Preventing the transmission of pathogenic microbes during
anesthesia. Expert Review of Medical Devices 2, 319-326.
75
