
A BIOMIMETIC AND BIOMECHANICAL
APPROACH FOR TISSUE ENGINEERING
Hybrid Nanomaterials and a Piezoelectric Tunable Bending
Apparatus for Mechanically Stimulated Osteoblast Cells Growth
Antonio Apicella and Raffaella Aversa
Material Lab, Dept. of Cultura del Progetto, Specialistic School of Industrial Design for Innovation,
Second University of Naples, Aversa, Italy
Keywords: Biomimetics, Biomechanics, Hybrid nanostructured biomaterials, Piezoelectric.
Abstract: The research develops and tests new hybrid biomimetic materials that work as mechanically stimulating
“scaffolds” to promote early regeneration in implanted bone healing phases. A biomometic nanostructured
osteoconductive material coated apparatus is presented. Bioinspired approaches to materials and templated
growth of hybrid networks using self-assembled hybrid organic-inorganic interfaces is finalized to extend
the use of hybrids in the medical field. Combined in vivo, in vitro and computer-aided simulations have
been carried out. Such multidisciplinary approach allowed us to explore many novel ideas in modelling,
design and fabrication of new nanostructured biomaterials and scaffolds with enhanced functionality and
improved interaction with OB cells. In vivo tests of Titanium screw implanted in rabbit tibiae have shown
that mechanical stimulation was induced by the presence of bioactive hybrid perimplantar scaffold resulting
in a differentiation and development of mesenchymal tissues. In order to investigate the relationship
between bone growth and applied mechanical loading (strain), a piezoelectically driven cantilever and a
computer-controlled apparatus for "in vitro" tests has been developed and presented.
1 INTRODUCTION
Developing innovative tissue engineering
biomimetic materials based on hydrophilic polymers
has been extensively studied in the past decades on
their physical, biological and mechanical properties.
Although hard in the dry state, such polymers swell
in water turning to soft and flexible materials used in
several biomedical applications such as ophthalmic
lenses, vascular prostheses, drug delivery system
and soft-tissue replacement (Montheard et al., 1992;
Apicella et al., 1993). Improved cytocompatibility in
terms of cell adhesion and metabolism for IPN of
HEMA and PCL was explained in terms of
increased surface hydrophobicity leading to
improvement of cell adhesion and spreading
(Schiraldi et al., 2004). Highly biocompatible novel
hybrid materials based on fumed silica and
hydrophilic poly-(hydroxy-ethyl-methacrylate)
(pHEMA) have been developed by the authors
(Schiraldi et al., 2004). The addition of fumed silica
is expected to improve the organization of the
polymeric network promoting hydrogen bonding of
the polymeric chains with the hydrophilic
nanoparticles. The resulting nanocomposites
consisted in more rigid transparent materials with
surprisingly improved mechanical strength and
cytocompatibility (Schiraldi et al., 2004) that
overcome one of the major drawbacks in hydrogels
applications associated with their poor mechanical
strength. Early studies confirmed that the nanofilled
hybrid composites possess biomimetic and
osteoconductive properties that can be useful in the
design of mechanically bioactive innovative
scaffolding systems for Osteobast (OB) growth
(Schiraldi et al., 2004). In healthy conditions,
modelling and remodelling collaborate to obtain a
correct shape and function of bones. This condition
is completely altered when bone is implanted with a
rigid prosthesis (Aversa et al., 2009; Sorrentino et
al., 2007). Loads on bones cause bone strains that
generate signals that some OB cells can detect and
respond to. Threshold ranges of such signals are
genetically determined and are involved in the
280
Apicella A. and Aversa R..
A BIOMIMETIC AND BIOMECHANICAL APPROACH FOR TISSUE ENGINEERING - Hybrid Nanomaterials and a Piezoelectric Tunable Bending
Apparatus for Mechanically Stimulated Osteoblast Cells Growth.
DOI: 10.5220/0003887902800285
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 280-285
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

control of modelling and remodelling (Sorrentino et
al., 2007; Frost, 1990; Wolff, 1892; Frost, 1964;
Frost, 1994). Early studies by Wolff (1892) stated that
mechanics could determine changes in the
architecture of bones (Wolff, 1892). In 1964 Frost
expressed mathematically the reactions of the bone
tissue to given stimuli to quantitatively assess bone
deformations (Frost, 1964). Remodelling processes
repair the damage removing and replacing the
damaged tissues with new bone. Moreover,
overloading (or under-loading) alters such
phenomenon (Frost, 1994). Mechanically compatible
hydrogels as scaffolding materials could increase
prosthesis adaptation mechanisms introducing active
interfaces that improve implant biomimetics while
reproducing cartilage and ligaments bio-mechanical
functions. Adaptive properties of bone benefit of use
of biomimetic (biomechanically compatible and
bioactive) scaffold bio-materials.
2 MATERIALS AND METHODS
Our Biomimetic and Biomechanical approach
resulted from a parallel mechanical and physical
characterization of new hybrid material coupled to
the bio-mechanical Finite Element analysis of the
biological system investigated (implanted bones).
The mechanics of the “in vitro dynamic bender
testing apparatus” were designed by using FEA
analysis utilising the material properties of the
swollen hybrid pHEMA based nanocomposites.
2.1 Materials
Commercial 2-hydroxyethyl methacrylate, was
purchased from Sigma-Aldrich Chemicals Co., (St.
Louis, MO, USA). Fumed silicon dioxide (Aerosil
300 Degussa, Germany) with a mean diameter of 7
nm and specific surface area of 300 m
2
⋅g
−1
was
utilized as the bioactive filler. The initiator, α-α’
azoisobutyrronitrile (AIBN), was purchased from
Fluka (Milan, Italy).
HEMA monomers were mixed with increasing
amount of fumed silica (4 to 30% by volume),
according to the procedures described in a previous
work. The resin was poured in 10 mm diameter
cylindrical moulds, polymerized in a forced air
circulation oven set at 60°C for 24 hrs and finally
postcured at 90°C for 1 h.
2.2 Sorption and Swelling Test
The cylindrical samples were used for the water and
isotonic saline (0.15 M NaCl) water solution
sorption and swelling experiments. The solution
uptakes were determined at equilibrium by
gravimetric measurements in a 0.1 mg Mettler
Toledo balance (Milan, Italy). The advancing
swelling fronts in the anomalous Case II (Apicella
and Hopfenberg, 1982) of the samples were
monitored measuring the thickness of the un-swollen
residual glassy core as a function of time.
The equilibrium sorption and swelling experiments
were performed at 37°C (thermostatic water bath)
until constant weight up-take was monitored (100 h).
2.3 Finite Elements Analysis
Finite Element Analysis (FEA) on models of the
Titanium implanted bones (human mandible
segment) and of the in vitro bender set-up was
performed according to the following procedures.
2.3.1 Models Set-up
Implanted bone (human mandible section)
The solid models were generated using Solidwork
2007 software. Titanium implant and the
surrounding part of a mandibular cortical and
cancellous bone were modelled. The average
anatomical dimensions of the maxillary bone were
generated according to literature data (Schwartz-
Dabney, 2003) as a cancellous core surrounded by
2.0 mm-thick cortical bone. The FE model was
obtained by importing the solid models into ANSYS
rel. 9.0 FEM software (Ansys Inc. Houston) using
IGES format. The volumes were meshed with eight
nodes brick with 3 degree of freedom per node,
resulting in a 3D FE model made up of 31,240
elements and 35,841 nodes. The model was
constrained at the top surface of the maxillary bone.
Accuracy of the model was checked by convergence
tests (Sorrentino et al., 2007).
Piezoelectric Bender
The geometry of the piezoelectric bender has been
measured on the commercial product (see section 2.5)
and transferred to the FE environment according to
the procedures described for the implanted bone. A 3
mm thickness symmetrical layers of rubber hydrogel
were modelled at the two piezoelectric bender
surfaces (simulating a thick coating of our swollen
pHEMA based hybrid composite)
2.3.2 Mechanical Properties of Materials
Orthotropic assumption for cortical bone was
adopted while the cancellous bone was considered as
isotropic linear materials. The Young’s modulus and
A BIOMIMETIC AND BIOMECHANICAL APPROACH FOR TISSUE ENGINEERING - Hybrid Nanomaterials and a
Piezoelectric Tunable Bending Apparatus for Mechanically Stimulated Osteoblast Cells Growth
281

Poisson’s ratio of isotropic materials used in the
models of the Titanium implanted bone and
piezoelectric bender are shown in Table 1
(Schwartz-Dabney, 2003).
Table 1: Isotropic mechanical properties of materials.
Young’s modulus Poisson’s ratio
Cancellous bone 0.91 (GPa) 0.30
Titanium 110 (GPa) 0.30
Piezoelectric ceramic 400 (GPa) 0.15
Swollen pHEMA Hybrid
nonocomposite
5 (MPa) 0.48
Cortical bone was divided into two sites, one on the
buccal side and one on the palatal side according to
the mechanical characterization reported for a
dentate mandible by Schwartz- Dabney and Dechow
(Schwartz-Dabney, 2003). Each area has its own
orthotropic constants values and orientation of the
maximum stiffness direction, the directions of the
maximum stiffness is referred to the occlusal plane.
E and G are expressed in GPa. Direction of
maximum stiffness are referred to the global
coordinate system. Orthotropic elastic constants and
orientation for buccal and palatal sides of cortical
bone are reported in Table 2.
Table 2: Orthotropic constants adopted for the cortical
bone on the buccal and palatal sides, respectively.
Max
stiff.°
E1 E2 E3 G12 G31 G23 _12 _31 _23
facial
39.9
11 15 18 4.5 4.7 5.7 0.21 0.25 0.42
buccal 4.4 12 18 19 4.9 4.9 5.1 0.16 0.31 0.43
Local orientation of the maximum stiffness (E3) and
the other two orthogonal stiffness directions (E1,
E2) have been reproduced dividing the shell of
external elements (compact bone structure) in
orientation sites according to the proposed
experimental mechanical characterization. The
orientation of the maximum stiffness has been
reproduced for each site by defining a local
coordinate system and by orienting the site’s
elements coordinate systems accordingly.
The maximum stiffness (E3) directions in degree
referred to the occlusal plane on buccal and palatal
side are reported in Table 2. E1 direction is normal
to the cortical surface.
2.4 Mechanical Characterization
Shear elastic modulus measurement on dry and
swollen p-HEMA Hybrid nanocomposites were
performed using a METTLERTOLEDO (Zurich,
Switzerland) dynamical mechanical tester operating
in shear mode (DMA). The elastic and viscous
components of the shear modulus were measured
under constant frequency loading in isothermal
condition. The samples were dried under vacuum at
a 60°C for 24 h before testing. In the shear test
mode, the 10 mm diameter and 2 mm thickness
sample disks are placed between three steel plates
forming a symmetrical sandwich. An isothermal
scan at 37°C in a dry Nitrogen purged environment
was performed. The deformation control was set to
10 μm and a force limitation of 0,9 N was applied at
an oscillating frequency of 10 Hz.
2.5 Mechanical Controlled Bender
57 mm piezoelectric benders (Quick-Mount 503,
PIEZO SYSTEMS, INC. Woburn, MA USA) were
used to build the oscillating dynamic scaffold
supports. A proprietary software and electronic
apparatus was used to drive oscillating output voltage
exit (0-100V). The deformations at the bender surface
were monitored by acquisition data software (System
4000 with 20 input channels by Vishay Measurements
Group Inc., NC, USA) at 2 points/s. The bender
operates with max displacement of 1.0 mm.
2.6 In Vivo Osteointegration Tests
2 implants for each rabbit tibia, in 6 rabbits (total of
24 implants in New Zealand White rabbits); each
rabbit has been implanted with 2 implants coated
with the nanostructured hybrid biomaterial on the
right tibia and 2 control implants in the left tibia.
Micro Computer Tomography with resolution 1
voxel=15 cubic micron has been performed on the
explanted tibiae after 1 week, 1, 2, 4 and 6 months.
BV/TV (Bone Volume/Total Volume), BS/TV
(Bone Surface), TbTh (Trabecular Thickness), TbSp
(Trabecular Separation), TbN (Trabecular Number)
have been used to evaluate the Total BIA (bone
implant apposition).
3 DISCUSSION
The aim of our research was to develop a
biomimetical/biomechanical approach for the design
of the experimental dynamic procedures (biological-
mechanical stimulus) finalized to favour adaptive
directionally organized OB growth in vitro scaffold
mineralization. In order to achieve this result, both a
proper biomimetic scaffolding material and an
externally driven mechanically straining apparatus
have had to be designed.
The biomimetic characteristic of our hybrid
materials have been investigated both for
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
282
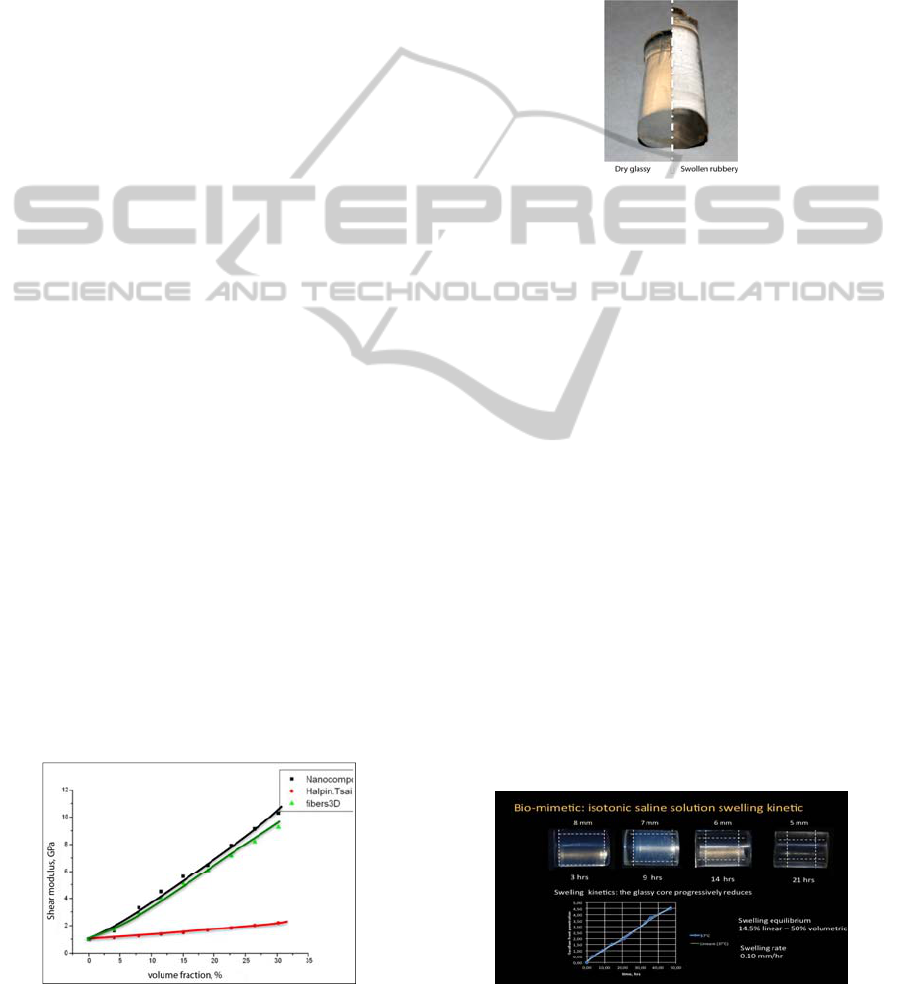
mechanical than osteoconductive properties.
3.1 Bio-mimetics: Hybrid
Nanocomposites Properties Design
Physiological bone material behaviour to be
mimicked by the bio-active scaffolding material
relates to the following aspects:
- mechanical properties (dry – swollen)
- bioactivity (in vivo tests)
In order to define the proper nanofiller/polymer ratio
of potentially idoneous the hybrid nanocomposites
The objective properties requirements are
- similar to bone rigidity (Elastic Modulus 6-15
GPa and shear modulus G 2-5 GPa) when dry
- similar to cartilage and ligament flexibility
(Halpin and Kardos, 1976) (high deformability -
Elastic modulus 2-20 MPa) when swollen.
3.1.1 Mechanical Properties
The dry Hybrid pHEMA nanocomposites with
compositions ranging from 4 to 30% by volume of
nanosilica were isothermally shear tested in a
Dynamic Mechanical Analyser operating at 10Hz
and at 37°C. The samples showed a predominantly
elastic behaviour (the viscous component was
negligible for all compositions). The values of the
measured Shear moduli are reported in figure 1.
The compositional dependency of the shear
modulus of the nanocomposites does not follow the
ordinary Halpin-Tsai relationship utilized to describe
the elastic properties of particulate composites (red
line) (Töyräsa et al., 2001), while resembles to that
of a 3D oriented fibre mat (green line in figure 1),
indicating the formation of a continuous hybrid
ceramo-polymeric structure. Shear moduli
comparable to those of the cortical bone have been
measured for nano-silica volumetric fractions
ranging from 4 to 12%. A volume fraction of 5% has
been then chosen for the in vivo osteointegration
tests and for the FEA simlations.
Figure 1: Hybrid shear moduli (black), Halpin-Tsai (red)
and 3D fibres composite (silica glass fibres mat) (green).
3.1.2 Swelling and Sorption Behavior
The 5% hybrid nanocomposites dramatically swell
in water solutions (figure 2) picking up 50% of its
dry weight and reducing its shear modulus to 2-3
MPa (measured in the DMA). Such phenomenon is
associated to the water induced polymer
plasticization that reduces the polymer glass
transition temperature below the test temperature
Figure 2: Swelling behaviour of the nanostructured hybrid
scaffold material.
This behaviour has been investigated in a
physiological isotonic 0.15 M NaCl solution held at
37°C for the 5% volume fraction sample both for
equilibrium and swelling kinetic. Once exposed to
the water solution, the initially dry and glassy
pHEMA composite starts to swell showing a clear
front dividing the rubber swollen external portion
and the unaffected glassy core. This glassy core
thickness progressively reduces as the swollen front
advance through the sample (upper part of figure 3).
A measure of the swelling kinetic is given by the
rate of reduction of the glassy core as a function of
the time (lower diagram in figure 3). The swelling
front advances at constant rate: this behaviour is
characteristic of a limiting relaxation controlled
sorption mechanism indicated as “Case II sorption”
(Apicella et al., 1993; Schiraldi et al. 2004; Aversa
et al., Sorrentino et al., 2007; Frost, 1990; Wolff,
1892; Frost, 1964; Frost, 1994; Apicella and
Hopfenberg, 1982). At equilibrium, when swelling
fronts meet, a 14.5% increase of the sample diameter
has been measured (about 50% of volume increase).
The resulting swelling rate is of 0.10 mm per hr.
Figure 3: Swelling kinetic of a 5% by volume hybrid
nanocomposite in 0.15M NaCl water solution (isotonic).
A BIOMIMETIC AND BIOMECHANICAL APPROACH FOR TISSUE ENGINEERING - Hybrid Nanomaterials and a
Piezoelectric Tunable Bending Apparatus for Mechanically Stimulated Osteoblast Cells Growth
283
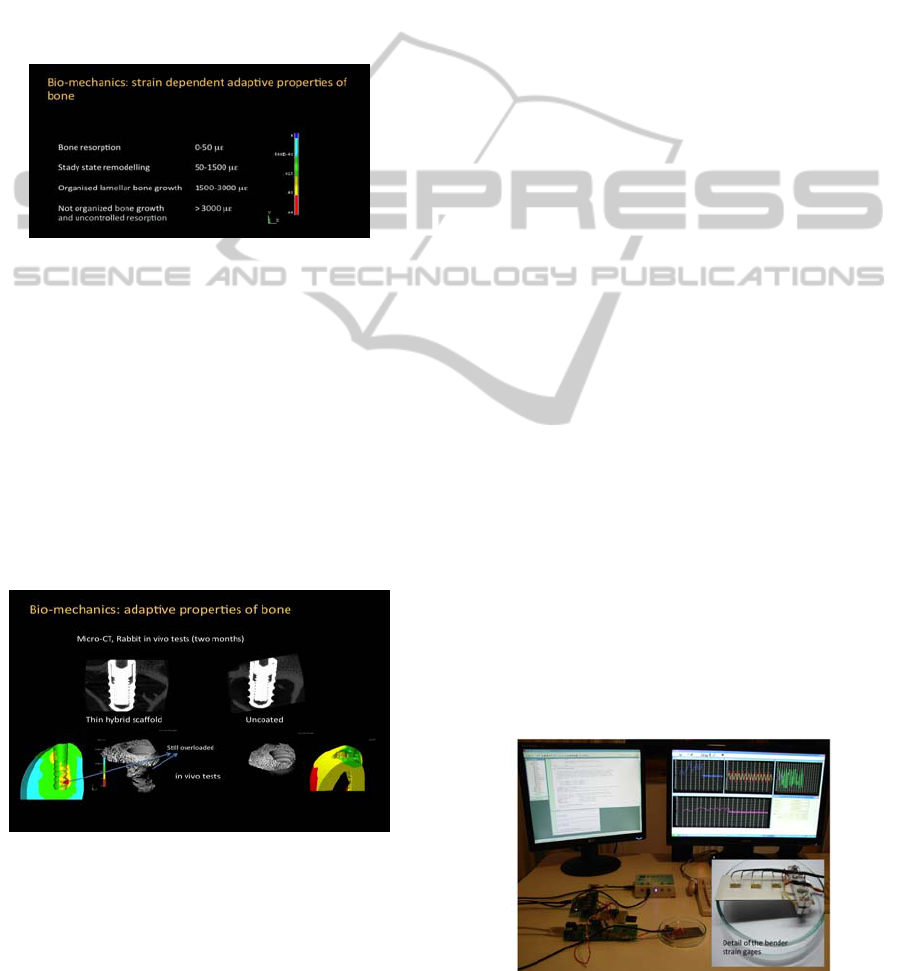
3.2 Bio-mechanics: Adaptive
Properties of Bone
The use of biocompatible and biomechanically
active interface that can be “designed” to reproduce
bone compatible and biomimetic strain distribution
is discussed in the present paper. The ranges of the
physiological strains and related bone adaptive
properties according to (Frost, 1990; Frost, 1994) are
reported in figure 4. There are upper (>3000με) and
lower (< 50με) strain limits that do not favour
healthy bone growth.
Figure 4: Adaptive properties of bone: strain ranges for
bone resorption (< 50με blue), remodelling (50-1500
με light blue-green) and organized growth (1500-
3000με yellow-red), resorption (> 3000με).
The comparison between FEA simulation of
physiological strains in coated and uncoated
implants and bone volumes in the in rabbit tibiae
after two months of in vivo test is reported in figure
5. The FEA simulations of the strain distribution
reported of the same figure 5 have been run on a
mandibular bone section that presents comparable to
the rabbit tibia mechanical and dimensional
characteristics.
Figure 5: Osteointegration of Titanium implants with a
nanostructured hybrid biomimetic coating (left side) and
without (right). Micro-CT bone reconstruction and FEA
calculated physiological strain distributions are compared.
The Micro CT bone reconstructions of the
perimplantar areas of the hybrid nanostructured
biomaterial coated and of the “as received” implants
after two months are compared in figure 5: the
nanostructured biomaterial coated implant shows a
better osteointegration that can be related to the
osteoconductivity of the perimplantar biomimetic
hybrid coating.
The bone implant apposition or bone ingrowth
(COMERON, 1986), which is defined as the
percentage of osteointegrated implant length for the
biomimetically coated and uncoated implants in the
six months in vivo test show a significant
improvement of about 100% increase in the first two
months and 30% after 6 months.
The Osteoblast proliferation and bone growth in
the implanted tibiae is clearly favoured and
accelerated by the presence of the hybrid
nanostructured coating. The biomechanical approach
using the adaptive properties of bone well describes
the biomimetic behaviour of the proposed
perimplantar hybrid scaffold since it can predict
areas of bone resorption (FEA model elements with
strains below the physiological lower limits have
been removed in the image), as it occurs in the in
vivo tests at the neck of the implant (Micro CT
reconstruction on the right side of figure 5). The
proposed biomechanical model can predict areas of
bone growth (FEA model and micro CT
reconstruction compared in the left side of figure 5).
According to Frost:
1. Remodelling is triggered not by principal
stress but by ’strains’.
2. Repetitive dynamic loads on bone trigger
remodelling while static loads do not.
Dynamic factors have been accounted and
utilized to design a piezoelectric driven dynamic
scaffold deformation apparatus for in vitro adaptive
osteoblast cells growth. Biomimetic aspects are
invesigated by using the osteoconductive hybrid
nanocomposites bender thick swollen coating
coupled with a FEM modelling of the in vitro bone
adaptive growth. The piezoelectric dynamic bender
and control system are shown in figure 6.
Figure 6: Piezoelectric bender for dynamic OB cells
culture tests: calibration test configuration.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
284

FEA results showing the distribution of the
unidirectional strains in the x direction (EPSX) in
the simulation of 1 mm oscillation at the free end in
the controlled bending test is reported in figure 7.
In the red-orange zone (1500 microepsilon), the
strains are compatible with those inducing organized
lamellar bone growth in healty bone. OB
colonization of the hybrid scaffold in the areas of
biologically compatible straining is the favoured.
Figure 7: FEA for the evaluation of the unidirectional
strains in the oscillation controlled bender.
The organized bone to growth in the red-orange
area causes a localized increase of the ossified
scaffold stiffness. Considering these localized time
dependent stiffening in the material highly strained
areas, a bone growing front moving in the direction
of the arrow (figure 8) is generated.
The rigid growing bone front lets a new area of
the scaffold to be bio-actively stimulated according
to the physiological strain for steady state
remodelling (50-1500 με - light blue and green
areas) and organized lamellar bone growth (1500-
3000 με - yellow-red areas). The oscillating
straining apparatus, than, can be used in in vitro
experiments to bone grow in thick scaffolds from
OB cell culture (figure 8).
Figure 8: Dinamic FEA simulation of the advancing bone
growth front under dynamic flexure straining conditions.
4 CONCLUSIONS
A biomimetical/biomechanical approach has been
pursued in designing the experimental dynamic
procedures (bio-mechanical stimuli) for in vitro
scaffold mineralization and ossification using
piezoelectric benders. The proposed material is a
Nanocomposites - Hybrid ceramo-polymeric poly-
Hydroxyl-Ethyl-Methacrylate (pHEMA) additioned
with nanosilica particles (4-6% by volume). This
biomimetic material swells in presence of
physiological solution (when in a biological aqueous
environment) picking-up to 50-30% by weight of
water. Mechanical behaviour in the glassy state is
comparable with bone while, in the swollen rubbery
state, is comparable with those of cartilage and
ligaments.
ACKNOWLEDGEMENTS
Funds from PRIN 2008 and FIRB (Funds for Base
Resarch) Futuro in ricerca 2008.
REFERENCES
Montheard JP, Chatzopoulos M, Chappard D. J Macromol
Sci Macromol Rev 1992;32:1–34.
Apicella A, et al. Biomaterials 1993;142:83–90.
Schiraldi C, D, Apicella A, Aversa R, De Rosa M (2004)
Biomaterials 25 (17):3645–3653.
Aversa R, Apicella D, Apicella A (2009). Dental materials
2009; 25: 678–690
Sorrentino R, Aversa R, Apicella A. Dent Mater 2007; 23:
983–93.
Frost HM. Anat Rec 1990; 226:403–13.
Wolff J. Das Gesetz der Transformation der Knochen.
Berlin: A Hirschwald; 1892.
Frost HM. Mathematical elements of lamellar bone
remodeling. Springfield: C. C Thomas; 1964. pp. 22–25.
Frost HM. Angle Orthod 1994; 64:175–88.
Apicella A, Hopfenberg Hb. Journal of Applied Polymer
Science, 1982; Vol. 27(4), P. 1139-1148, Issn: 0021-8995
Schwartz-Dabney, C.L. (2003) American Journal of
Physical Anthropology 120: 252-277.
J Töyräsa, et al., Journal of Biomechanics, Volume 34,
Issue 2, 2001, 251-256
J.C. Halpin and J. L. Kardos; Polymer Engineering and
Science, 1976, v16, N5, pp 344-352
H. U. COMERON, Clin. Orthop. 208 (1986) 81
A BIOMIMETIC AND BIOMECHANICAL APPROACH FOR TISSUE ENGINEERING - Hybrid Nanomaterials and a
Piezoelectric Tunable Bending Apparatus for Mechanically Stimulated Osteoblast Cells Growth
285
