
Brain Computer Interface and Eye-tracking for
Neuropsychological Assessment of Executive Functions:
A Pilot Study
Pietro Cipresso
1,*
, Paolo Meriggi
2
, Laura Carelli
3
, Federica Solca
3
, Barbara Poletti
3
,
Dorothée Lulé
4
, Albert C. Ludolph
4
,Vincenzo Silani
3
and Giuseppe Riva
1
1
Applied Technology for Neuro-Psychology Lab, IRCCS Istituto Auxologico Italiano
Via G. Pellizza da Volpedo, 41, 20149, Milano, Italy
Polo Tecnologico – Biomedical Technology Department
Fondazione Don Carlo Gnocchi Onlus, Via Capecelatro, 66, 20148, Milano, Italy
3
Department of Neurology and Laboratory of Neuroscience, “Dino Ferrari” Center
Università degli Studi di Milano, IRCCS Istituto Auxologico Italiano
Piazzale Brescia, 20, 20149, Milano, Italy
4
Department of Neurology, University of Ulm, Oberer Eselsberg 45, 89081, Ulm, Germany
Abstract. In this study we explored the use of Brain Computer Interface (BCI)
and Eye-Tracking (ET) technology both as augmentative and alternative
communication (AAC) tool and to assess cognitive deficits. Specifically, we
focused on the possible development of a neuropsychological battery for
cognitive assessment based on the integration of BCI and ET tools. To
preliminary test this approach we assessed eight healthy subjects with a
widespread used cognitive task. AAC and usability of both instruments have
also been evaluated with the aim to fine-tune the overall system architecture for
clinical use.
1 Introduction
Some of the most consistently reported cognitive changes regards frontal executive
functions, e.g. verbal fluency, attention, working memory, planning and abstract
reasoning [1-6]. However, the assessment of cognitive impairment still remains a
problematic issue in patients, because of the possible presence of severe physical
disabilities, including movement impairment, paralysis in the advanced stages and
dysarthria, which interfere with the outcome of traditional neuropsychological testing.
New technologies to enable communication have been recently used in several
studies; however, a comprehensive battery for cognitive assessment has never been
implemented with these promising methodologies. Among these methods, Brain
Computer Interface (BCI) and Eye Tracking (ET) are the most promising
Cipresso P., Meriggi P., Carelli L., Solca F., Poletti B., Lulé D., C. Ludolph A., Silani V. and Riva G..
Brain Computer Interface and Eye-tracking for Neuropsychological Assessment of Executive Functions: A Pilot Study.
DOI: 10.5220/0003893100790088
In Proceedings of the 2nd International Workshop on Computing Paradigms for Mental Health (MindCare-2012), pages 79-88
ISBN: 978-989-8425-92-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

technologies. BCI uses neurophysiological signals as input commands to control
external devices, while ET allows the measurement of eye position and movements.
To date, no applications have been developed, using ET as a communication
device in order to administer traditional cognitive tasks to patients.
The main disadvantage in the use of ET systems is that they require good ocular
mobility, and the absence of important visual deficits; the former may be lost or
altered in the final stages of disease, and the latter may be present in patients of
advanced age, thus forbidding the use of this device. In fact, ET may not be
proficiently used in case of poor or lack of eye-motor control, such as in late stages of
the disease. In this case there is the need of a more direct interface between voluntary
cortex activity and the computer. BCI may offer an interesting answer to this issue
with a growing number of different paradigms proposed. The most frequently used is
the P300, an event related potential (ERP) elicited by infrequent task stimuli, that
occurs 200-700 ms; it is typically recorded over central-parietal scalp locations [8-10]
and it can also be used by patients suffering from complete paralysis and impairment
in oculo-motor dysfunctions, such as locked-in patients.
It is notable, however, that 20% of subjects are not proficient in using BCI; this
phenomenon is called “BCI illiteracy” [11] and it is due to the fact that some users do
not produce brain activity detectable at the scalp level, independently from the health
conditions: even about 10% of healthy subjects do not produce “usable” P300.
With regard, as an example, to Amyotrophic lateral sclerosis (ALS) patients,
studies have shown that some of them produce less typical ERPs than healthy
matched subjects [12]; [13]. A previous ERP study in patients with sporadic ALS
found that P3a and P3b amplitudes of ALS patients were lower and P3a latencies
were significantly longer compared with the controls [14]; ERP recordings in non-
demented patients with sporadic ALS also showed prolonged N200 and P300
latencies compared to healthy controls [15]. Ogawa and colleagues [16], by
employing neuropsychological measures, event-related potentials (ERPs) and clinical
scales, studied a sample of patients with early-stage sporadic ALS. They found that
patients with the bulbar-onset type showed marked prolongation of P3 latency
compared to patients with the limb-onset type and controls. Furthermore, bulbar
functional rating scale correlated with prolonged P3 latency and low P3 amplitude.
Additionally, patients with bulbar-onset ALS had consistently poorer cognitive test
performance than those with limb-onset ALS [17]. These results represent a challenge
for the use of P300 as an input signal in BCIs. Kübler and Birbaumer [7] investigated
the relationship between the level of motor and physical impairment and the ability to
use brain computer interface by comparing three different BCI systems (P300, SCP
and SMRs, i.e. sensorimotor rhythms). They found no continuous decrement in BCI
performance with physical decline, even in the completed locked-in state (CLIS)
where no communication was possible.
Two important criteria in order to evaluate the feasibility of a BCI system are
speed and accuracy [18]. The former is related to the fact that the more rapidly a BCI
can be controlled, the greater quantity of information can be produced by the user and
the greater the chance for effective communication. Obviously, compared to verbal
speech production, communication rate is severely reduced with BCI. With regard to
accuracy, it consists of the percentage of correct selections per time interval. A wrong
selection could turn into an error in communication, with both practical and
psychological consequences for the user. In order to avoid this, the BCI system must
80

be equipped with options that allow the user to correct wrong selections. A balance
between speed and accuracy should be identified.
A recently funded project, “eBrain: BCI-ET for ALS", [19] aims to evaluate BCI
P300 technique and eye-tracking technology both as AAC systems and as cognitive
assessment tool with ALS patients.
Before beginning the actual testing phase with ALS patients, we performed a pilot
study with healthy subjects to fine-tune the overall project testing setup. Specifically,
a small group of healthy subject was administered a subset of the eBrain designed
sessions to generally assess feasibility, user-friendliness, pleasantness and fatigue.
Emotional aspects related to the experimental setting, as well as its usability, have
been evaluated, too. In this paper we report the results of this pilot study.
2 Materials and Methods
2.1 Subjects
Eight healthy subjects (4 females and 4 males) constituted the participants, ranging in
age between 25 and 39 (M: 31.75, SD: 5.946). They were all volunteers with a
schooling degree ranging from 13 to 24 years of education (M: 19, SD: 4.276). All the
subjects were experienced in the use of PC (50% fair or good and 50% excellent),
some of them (50%) declared to have already used Brain Computer Interface or an
Eye-tracker system, and more than half (62.5%) had already participated into EEG
experiments. Exclusion criteria were related to the states of participants’ cardiac,
optical, mental, and psychological health. Participants were asked not to drink
caffeine or alcohol and not to smoke prior to the experimental test to avoid any effects
of these substances on the central and autonomic nervous system.
2.2 System Setting
Test architecture (Figure 1) was composed of an eye-tracking system, and a BCI
device, both controlled by a laptop PC (HP DV3-4101SL, Hewlett Packard, USA),
connected to an external monitor, meant for the stimuli presentation (Display PC).
The BCI device module consisted of a g.USBAmp biosignal amplifier (Guger
Technologies, Graz, Austria), connected to an active electrodes head cuff
(g.GammaCap, Guger Technologies). 16 EEG channels were used (FZ, C3, C4, CZ,
CPZ, P3, P4, PZ, PO3, PO4, POZ, PO7, PO8, O1, O2, OZ); ground was placed in
FPZ, and reference was located on the left ear lobe.
The eye-tracker was an Eyelink-1000 (SR Research Ltd., Mississauga, Ontario,
Canada), consisting of a high-speed infrared camera and the related illuminator,
positioned just below the Display Monitor.
Tests were administered through two different software: a customized version of
BCI2000 (http://www.bci2000.org/BCI2000/Home.html) [41]; [42] for the BCI
assessment and a suitable custom software, developed within the project for the eye-
tracking evaluation.
81

2.3 Experimental Procedures
Subjects who met the experimental criteria were enrolled and tested at the Applied
Technology for Neuro-Psychology Laboratory, located at the Istituto Auxologico
Italiano in Milan. All subjects were required to sign a release form. Since the
experiment was constituted by two separated parts, the design was balanced between
the subjects. Immediately after the former session, subjects were asked to fulfill the
required questionnaires and to rest for 15 minutes.
After a calibration, required by both instruments, the neuropsychologist started the
cognitive assessment through a Phonemic Fluency test and a Semantic Fluency test
(as described below), adapted to the experimental conditions (see figure 1).
At the end of Eye-tracking session, the subjects had to copy a sentence twice
(FIAT ALAN FORD MERCEDES BENZ): the first time using a virtual keyboard
(ordered letters in rows from A to Z see figure 2a), the second time using a scrambled
keyboard (mixed position of the letters, see figure 2b). The total time of the
experiment was about two hours.
Fig. 1. Experimental procedure timeline for both BCI and ET sessions.
2.4 Psychological and Usability Self-report Questionnaires
2.4.1 STAI form Y Questionnaire
The Italian version of the STAI form Y questionnaire was used to assess changes in
two different types of anxiety, namely, anxiety detected as the subject's current state
(STAI-Y1, i.e. state anxiety) and anxiety detected as a reasonably stable trait of the
personality of an individual (STAI-Y2, i.e., trait anxiety). A total of four Self-reported
STAI-Y1 were gathered before and after both BCI and Eye-tracking sessions. One
self-reported STAI-Y2 was gathered five minute before the experimental session [22].
2.4.2 Self Assessment Manikin (SAM)
The Self Assessment Manikin (SAM) is a non-verbal pictorial assessment technique
that directly measures the pleasure, arousal, and dominance associated with subjects'
affective reactions [23]; [24]; [25]. A total of four Self-reported SAMs were gathered
before and after both BCI and Eye-tracking sessions.
82
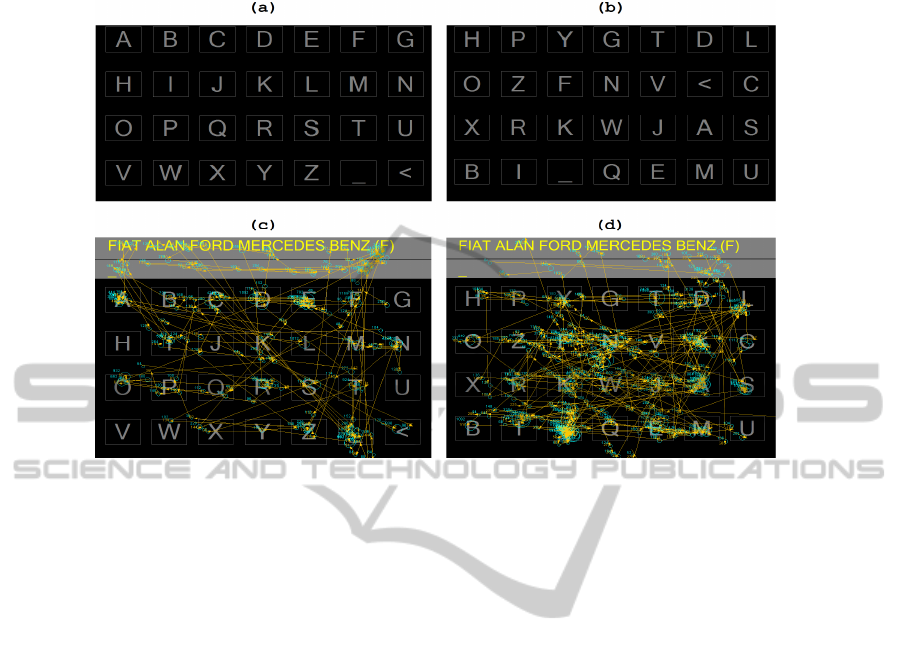
Fig. 2. The used ET Keyboards and the Saccades plotted over them: (a) Standard Virtual
Keyboard, (b) Scrambled Keyboard, (c) Saccades on Standard Virtual Keyboard, (d) Saccades
on Scrambled Keyboard.
2.5 Usability Inventory Post-test Questionnaire
Since there are no usability validated tests for Eye-tracking and BCI systems, we
developed a questionnaire with 19 items based on the literature available [26-29]. Our
purpose was to evaluate the instruments' general usability, and the following specific
variables: fatigue, screen readability, perceived usefulness, and so on.
2.5.1 Neuropsychological Tests
We used a classic spoken letter-based word generation procedures (Phonemic
Fluency), such as the Controlled Oral Word Association test [30-34] and then
repeated the procedure with the Category Fluency, also known as Semantic Fluency
[30]. These tests have been recognized as the most sensitive tools in detecting
cognitive impairments in ALS patients [5].
For both the Semantic and the Phonemic Fluency in the BCI session we used to
measure the time taken by the subjects to think the word starting with the given
letters: “A” and "H" respectively. A timer was started by the researcher right after the
letter was presented to the subject. Then, the timer was stopped when the subjects
indicated to be ready to effectively start to write the word with the BCI system.
Finally the procedure was repeated for the Semantic Fluency, with the categories
”furniture” and "means of transport."
Concerning the Eye-tracking system we used an adapted version of Fluency Index
[6] to adjust for eye motor component. Thus the subjects were required to write all the
83

words starting with a specific letter (“Q” and “Z”) in exactly one minute (generation
condition). Then the subjects were asked to copy exactly the same words while a
researcher measured the time taken (control condition). The same procedure was
repeated for the Semantic Fluency task, with the categories “type of shoes” and
“cooking ingredients.”
The difference between the specified time for the generation condition and the
time taken for control condition was then calculated and used to determine the
Fluency Index, which represented the average time taken to think about each word
[6].
3 Results
Data were analyzed with the aid of the statistical software SPSS, version 17
(Statistical Package for the Social Sciences–SPSS for Windows, Chicago, Illinois,
USA). Due to the small sample size, nonparametric tests were preferred, even if
several measures showed a normal distribution (also according to Kolmogorov-
Smirnov test).
In the following paragraphs the main results of this preliminary study are
presented.
3.1 Fluency Tests
In BCI session, Phonemic Fluency average time in seconds was 6.42±2.76 and
Semantic Fluency average time in seconds was 4.08±1.94. Regarding Eye-tracking
session, we used the Fluency Index, as above described. Phonemic Fluency Index was
4.28±5.84 and the Semantic Fluency Index was 3.37±2.58. These data will be used to
assess future patients’ performances.
3.2 Behavioral Measures
Wilcoxon Signed Ranks Tests indicated no statistical differences for both the pre-post
STAI-Y1 and pre-post SAM scales, indicating that no negative affective state or
anxiety have been generated by the performance with BCI or ET. However a small
increase in anxiety was detected after the use of BCI.
3.3 Usability
As can be seen in Table 1, subjects recognize both systems as enough usable, but Eye-
tracker is perceived as more usable than BCI (statistical significance is calculated
with Wilcoxon Signed Ranks Tests).
84
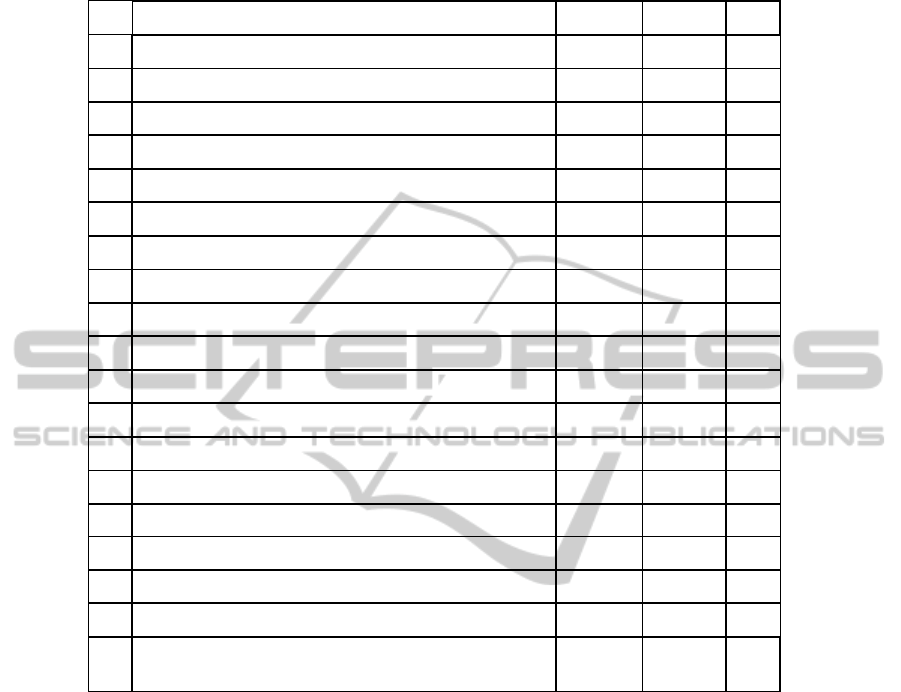
Table 1. Average values of 7-point Likert scale items of the usability questionnaire.
ID Item Mean BCI Mean ET p
1 It is easy to use the device 5.25 6.25 .131
2 The instructions are clear 6.25 6.50 .157
3 Sometimes I wondered if I was selecting the right letter 2.25 1.75 .336
4 Letters on the screen are clear and sharp 5.25 6.88 .066
5 I felt in command of this device when I was using it 5.13 5.38 .916
6 I properly understood the instructions 6.63 6.63 1.000
7 Using this device was frustrating 2.38 1.88 .357
8 I felt tense at times when using this device 2.63 2.50 .931
9 The device requires too many steps to work 2.00 1.38 .197
10 New users will find this device easy to use 4.63 6.25 .038
11 It is easy to make the device do what I want 5.13 5.38 .666
12 The device did what I expected 5.25 5.63 .732
13 The device appears to be limited 2.88 2.38 .480
14 The equipment is comfortable 3.75 4.63 .167
15 Using the device was demanding and tiring 4.13 2.63 .071
16 The icon to correct answers was helpful (ET) - 5.88 -
17 An initial tutorial on the usage of the device would be helpful 3.25 2.75 .194
18 Using the device was amusing and exciting 4.25 5.25 .071
19
I believe that the device is fit to communicate in the presence
of disorders that prevent from communicating with the voice
5.13 6.75 .102
3.4 Anxiety Traits and Consequences
No correlations were found between STAI-Y2 and all the Fluency measures, for both
the BCI and the Eye-tracking.
On the other hand we found interesting correlation between STAI-Y2 and some
items of the usability questionnaire. In particular, the item 15 for the BCI (the
instruments has been demanding and tiring) is positive correlated with STAI-Y2 (r =
0.747, p = .033). In sum, usability is negatively correlated with the trait anxiety level.
3.5 Copying Text using Different Keyboards with ET
The average time to copy the same text with the standard virtual keyboard was
46.09±6.55. The average time for the same copy with the scrambled keyboard was
85

60.17±21.23. Wilcoxon Signed Ranks Tests indicated statistical significant
differences (Z = -2.201, p = .028).
4 Conclusions and Future Work
No studies have been performed so far to evaluate BCI and the eye-tracking system
for AAC and cognitive assessment in ALS. Our pilot study provided evidences for the
effectiveness and usability of these techniques. Specifically, the BCI/computerized
assessment could provide new insight into the understanding of cognitive deficits,
when applied to ALS patients, through the integration of multidisciplinary data:
neurophysiological, neuropsychological, behavioural and psychological.
The proposed study is characterized by at least two innovative aspects: (1) the
comparison between two promising technologies, one already extensively
investigated (ET), the other being a very promising candidate (P300 BCI), (2) the
adaptation of a computerized verbal fluency task for the neuropsychological
assessment of higher order cognitive functions in ALS patients.
Results showed a good usability of both instruments, better for Eye-tracking, but
promising for BCI too. Furthermore the strong negative correlation between trait
anxiety and perceived usability clearly shows that the higher the subject is anxious,
the more the instruments will be perceived as demanding, tiring, and difficult to use.
Finally, no affective effects on cognitive performances were revealed by the
psychological measures administered.
As expected, BCI calibration was a critical issue. The average score obtained after
the calibration process was 89.73%, leading to an accuracy of BCI system during the
tests of 81.72% (i.e. 18.28% of errors). However, the 6 subjects that obtained 100% of
correct calibration did very few errors in the testing phase. These data suggest that it
is crucial to extend the calibration phase in order to reach very high correct ratio
(close to 100%).
More, the choice of virtual keyboard used in the task, clearly influences the
performances obtained, showing a high potential use of such instruments for the
development of novel cognitive tests.
Finally, these preliminary results may have interesting implications for both
clinical practice (the availability of an effective tool for neuropsychological
evaluation of ALS patients) and ethical issues, the last one arising from a proper
assessment of cognitive ability preservation, in particular regarding relevant decisions
about medical treatments, economical and end-life issues.
Acknowledgements
This study has been made possible partially due to funds from the Lombardy Region
project "eBrain: BCI-ET for ALS (eBrain: BCI-ET nella SLA)."
86

References
1. Bak T. H., Hodges J. R., (2001). Motor neurone disease, dementia and aphasia:
coincidence, co-occurrence or continuum? J Neurol, 248(4):260-70.
2. Strong M. J., Lomen-Hoerth C., Caselli R. J. et al., (2003). Cognitive impairment,
frontotemporal dementia, and the motor neuron diseases. Ann Neurol, 54:S20-23.
3. Strong M. J., Grace G. M., Freedman M., Lomen-Hoerth C., Woolley S., Goldstein L. H.,
Murphy J., Shoesmith C., Rosenfeld J., Leigh P. N., Bruijn L., Ince P., Figlewicz D.,
(2009). Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural
syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler, 10(3):131-46.
4. Zago S., Poletti B., Morelli C., Doretti A., Silani V., (2011). Amyotrophic lateral sclerosis
and frontotemporal dementia (ALS-FTD). Archives Italiennes de Biologie, 149: 39-56.
5. Phukan J., Pender N. P., Hardiman O., (2007). Cognitive impairment in amyotrophic lateral
sclerosis. Lancet Neurol, 6(11):994-1003.
6. Abrahams S., Leigh P. N., Harvey A., Vythelingum G. N., Grisé D., Goldstein L. H.,
(2000). Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS).
Neuropsychologia, 38(6):734-47.
7. Kübler A., Birbaumer N., (2008). Brain-Computer interfaces and communication in
paralysis: extinction of goal directed thinking in completely paralysed patients? Clinical
Neurophysiology, 119:2658-2666.
8. Fabiani M., Gratton G., Karis D., Donchin E., (1987). Definition, identification and
reliability of the P300 component of the event-related brain potential. Ackles, P.K.,
Jennings, J. R., Coles, M. G. H. (Eds.). Advances in psychophysiology, 2. New York, NY:
JAI Press, 1-78.
9. Sellers E. W., Donchin E., (2006). A P300-based brain–computer interface: Initial tests by
ALS patients. Clin Neurophysiol,117:538-548.
10. Sellers E. W., Kübler A., Donchin E., (2006). Brain-computer interface research at the
University of South Florida Cognitive Psychophysiology Laboratory: the P300. Speller.
IEEE Trans Neural Syst Rehabil Eng, 14(2):221-4.
11. Dickhaus T., Sannelli C., Muller K. R., Curio G., Blankertz B., (2009). Predicting BCI
performance to study BCI illiteracy. BMC Neuroscience, 10.
12. Nijboer F., Sellers E. W., Mellinger J., Jordan M. A., Matuz T., Furdea A., Halder S.,
Mochty U., Krusienski D. J., Vaughan T. M., Wolpaw J. R., Birbaumer N., Kübler A.,
(2008). A P300-based brain-computer interface for people with amyotrophic lateral
sclerosis. Clin Neurophysiol, 119(8):1909-16.
13. Paulus K. S., Magnano I., Piras M. R. et al., (2002). Visual and auditory event-related
potentials in sporadic amyotrophic lateral sclerosis. Clin Neurophysiol, 113:853–61.
14. Hanagasi H. A., Gurvit I. H., Ermutlu N. et al., (2002). Cognitive impairment in
amyotrophic lateral sclerosis: evidence from neuropsychological investigation and event-
related potentials. Cognitive Brain Research, 14:234-244.
15. Gil R., Neau J. P., Dary-Auriol M. et al., (1995). Event-related auditory evoked potentials
and amyotrophic lateral sclerosis. Arch Neurol, 52: 890-896.
16. Ogawa T., Tanaka H., Hirata K., (2009). Cognitive deficits in amyotrophic lateral sclerosis
evaluated by event-related potentials. Clinical Neurophysiology, 120:659-664.
17. Schreiber H., Gaigalat T., Wiedemuth-Catrinescu U., Graf M., Uttner I., Muche R.,
Ludolph A. C., (2005). Cognitive function in bulbar- and spinal-onset amyotrophic lateral
sclerosis. A longitudinal study in 52 patients. J Neurol, 252(7):772-81.
18. Kübler A., Kotchoubey B., Kaiser J. et al., (2001). Brain-Computer Communication:
unlocking the locked in. Psychological Bullettin, 127(3): 358-375.
19. Cipresso P., Meriggi P., Carelli L., Solca F., Meazzi D., Poletti B., Lulé D., Ludolph A. C.,
Riva G., Silani V., (2011), The combined use of Brain Computer Interface and Eye-
Tracking technology for cognitive assessment in Amyotrophic Lateral Sclerosis, 5th
87

International Conference on Pervasive Computing Technologies for Healthcare
(PervasiveHealth) (2011): p. 320-324.
20. Wolpaw J. R., Schalk G., McFarland D. J., Hinterberger T., Perelmouter J., Godde B.,
Birbaumer N., Pfurtscheller G., BCI2000: a general purpose brain–computer interface
system. Soc Neurosci Abstr 2000c;26:1229.
21. Schalk G., McFarland D. J., Hinterberger T., Birbaumer N., Wolpaw J. R., BCI2000:
development of a general purpose brain–computer interface (BCI) system. Soc Neurosci
Abstr 2001;27:168.
22. Spielberger C. D., Goursuch R. I. and Lushene R. E., (1970) Manual for state and trait
anxiety inventory (self-evaluation questionnaire). Consulting Psychologist Press, Palo Alto,
CA.
23. Lang, P. J., (1995). The emotion probe. Studies of motivation and attention. The American
Psychologist, 50(5), 372-385.
24. Lang P. J., Behavioral treatment and bio-behavioral assessment. In: Sidowski J. B., Johnson
J. H., Williams T. A., editors. Technology in mental health care delivery systems,
Norwood, NJ: Ablex, 1980, pp. 119–37.
25. Bradley, M. M. and Lang, P. J., (1994). Measuring emotion: The self-assessment manikin
and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry,
25, 49-59.
26. Nielsen, J., (1992). The usability engineering life cycle. IEEE Computer 25,3 (March), 12–
22.
27. Nielsen, J., (1993). Usability Engineering, Academic Press, Boston, MA.
28. Velichkovsky, B. and Hansen, J. P., (1996). New technological windows into mind: There
is more in eyes and brains for human-computer interaction. In Proc. CHI 1996, 496-503.
29. Kitamura, Y., Yamaguchi, Y., Imamizu, H., Kishino, F. and Kawato, M., (2003). Things
happening in the brain while humans learn to use new tools. In Proc. CHI 2003, 417-424.
30. Abe K., Fujimura H., Toyooka K., Sakoda S., Yorifugi S., Yanagihara T., Cognitive
function in amyotrophic lateral sclerosis. Journal of Neurological Sciences 1997;148:95-
100.
88
