
LeMo
Studying Chemical Molecular Structures through Gaming
Filipe Silva
1
, Tiago Alves
1
, José Braz
1
and Susana Piçarra
1,2
1
Escola Superior de Tecnologia de Setúbal, Instituto Politécnico de Setúbal
Campus do IPS, Estefanilha, 2910-761 Setúbal, Portugal
2
Centro de Química-Física Molecular and Institute of Nanoscience and Nanotechnology, IST/UTL
1049-001 Lisboa, Portugal
Keywords: e-Learning, Web Games, Learning through Gaming, Virtual Chemistry Lab, Lewis Structures, Molecular
Geometries, Polarity, Valence Shell Electron Pair Repulsion, VSEPR.
Abstract: The present work’s main contribution is the description of a first experiment to create a virtual chemistry lab
to be used at university level based on games instead of the traditional chemistry virtual labs based on
experiments. The paper starts with the motivation to use games as a teaching-learning process and with the
reason for choosing Lewis formulas and VSEPR theory to make a first experiment. Previous works, with
emphasis on web solutions for Chemistry and the role of emotions in the learning process are then
presented. The architecture of LeMo, the developed game, is described together with a detailed explanation
of its operation. The paper ends with a summary of the experiment’s results, which indicate that gaming
may be a good strategy for motivating students to study Chemistry.
1 INTRODUCTION
1.1 Motivation and Objectives
In order to improve students’ evaluation results, the
Chemistry Scientific Area within the Systems and
Informatics Department was interested in developing
a virtual tool that could allow students to practice
different contents. During the requirements analysis
and specification step of the development process,
the creation of a Chemistry Virtual Lab, that
students could use autonomously, was proposed.
After a brief study of the available e-learning
materials (Rekkedal et al., 2003); (Bottentuit et al.,
2006); (Espinilla et al.., 2010); (Allen et al., 2008,
2010) and of previous experiences within the
Chemistry Scientific Area it became clear that
several issues needed to be addressed prior to the
development of any serious experiment. To us the
most important issues were:
1. Which objectives should be considered for such
a platform: to improve the evaluation results? To
facilitate the learning and practice processes? To
give students an environment where they would
practice for longer periods?
2. Depending on the specified goals, a strategy had
to be defined. Three main options were identified:
cloning existing virtual labs, simply giving the links
to the best ones already present in cyberspace or
developing a new one.
The creation of an engaging environment was
established as the main objective. Considering that
the two first strategies had already been
implemented on the EST Setubal e-learning
platform
1
, and prior to engage in the development of
a full environment, we decided to test the strategy of
learning through gaming.
Taking the previous statement in consideration
we may say that the aim of the present application is
to provide a new method for practicing the writing
of Lewis formulas and applying VSEPR theory,
pointing students’ mistakes in real time and in a
pleasant way, in order to motivate students not to
give up when studying these subjects.
Note that only on-line and completely free of
charges solutions were considered during this work,
in order to minimize costs both for students and
institutions.
1
http://moodle.ests.ips.pt/ (accessed 2011/Dec/08) Please notice
that all links and Google queries quoted in the present work
where last accessed and reviewed on this same date.
506
Silva F., Alves T., Braz J. and Piçarra S..
LeMo - Studying Chemical Molecular Structures through Gaming.
DOI: 10.5220/0003934005060516
In Proceedings of the 4th International Conference on Computer Supported Education (SGoCSL-2012), pages 506-516
ISBN: 978-989-8565-07-5
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

1.1.1 Why Lewis Formulas and VSEPR
Theory
Molecular Structure is a compulsory chapter in any
introductory course of Chemistry. The most
elementary way to represent a molecule is using its
Lewis formula, which shows all chemical bounds
between atoms. However, and by itself, a Lewis
formula doesn’t give any information about the
geometry of the molecule, which determines several
properties of the corresponding material (boiling
point, viscosity, etc.). The easiest way to determine a
molecular geometry from a Lewis formula is by
applying the Valence Shell Electron Pair Repulsion
theory, VSEPR – a model used to predict the shape
of individual molecules based upon the extent of
electron-pair electrostatic repulsion. Despite the
simplicity of these two subjects, students frequently
find them boring, especially if their graduation areas
are not in Chemistry.
Usually, students study these subjects by solving
exercises on paper and comparing the obtained
structures and geometries with the ones reported on
the solutions section. This method is often
frustrating as one single mistake is enough to lead to
a completely wrong structure and/or geometry. As it
is not easy for a student to identify the mistake by
himself, he usually gives up.
The Chemistry Group first attempt to overcome
this problem was the implementation of an
interactive multi-choice questionnaire at the moodle
based EST Setúbal e-learning platform
1
. As the
available wrong answers were based on the students
most frequent mistakes, a tip popped out whenever a
wrong answer was given, helping the student to find
the right option.
Implemented at the Oxford Universal Virtual
Chemistry Lab (Table 1) there is an application for
attributing geometries to molecules (from a database
of 20 molecules). Whenever the answer is right, the
molecule 3D representation appears, helping the
student to visualize its molecular structure.
The existence of these two applications also
contributed for choosing Lewis formulas and
VSEPR theory as contents for the present game.
1.1.2 Why Gaming?
From the last two decades of the past century on,
brain sciences have developed deep studies on the
role of emotions in conscience (Damásio, 1994).
These studies changed the understanding of the
relations between perceptions, emotions and the
learning processes (Damásio, 1999).
During the Middle Ages, when no registration of
land properties existed, it was a common practice to
choose a boy to witness land transactions. At the
moment of the transaction and in the presence of the
full audience, the boy was strongly hit in the face. It
was empirically understood that the boy would
remember that day for all his life and would identify
the landlord, in case of conflict.
Nowadays this practice is no longer used.
Nevertheless it is still well accepted that living a
strong emotion contributes to a better memorizing
process. Indeed, (Damásio, 2010) has recently
proposed a brain perception and memorization
model based on the relation between perception,
images and emotions. For what this paper is
concerned, do consider the concept of “Images” as
in (Damásio, 2010), pg 36: “The brain maps the
surrounding world [including the whole human
body], as well as its own functioning. These maps
are experienced as images in our mind. So the term
“images” refers not only to visual images but also to
images that came from any other sense, like
audition, visceral or tactile as a few examples”.
It is also worth mentioning recent works in
Affective Computing, pioneered by (Picard, 1995).
According to (Shen et al., 2009), “the influence of
emotions on learning is still under-emphasized.
Recently, a growing body of literature [in Affective
Computing] has begun to espouse the central role of
emotion to any learning endeavor and outcomes,
especially in online learning”.
Although Affective Computing studies are still in
progress, it is possible to assume that gaming, that
raises emotional states, should improve students’
learning capabilities.
2 PREVIOUS WORKS
2.1 First Attempts
Considering the discussion and definition of e-
learning presented by (Tavangarian et al, 2004) we
may consider that the use of e-learning in Chemistry
began almost half a century ago. Indeed (Yoshimura,
2006) refers its introduction in Japan in the 1980’s.
In USA it is consensual that PLATO
2
(Programmed
Logic for Automated Teaching Operations),
developed in the 1960’s, was pioneer in computer
assisted education. The earliest references to its use
on Chemistry can be found in (Smith, 1970) and
(Shacham et al., 1981). In Europe, and according
2
http://en.wikipedia.org/wiki/PLATO_(computer_system)
LeMo-StudyingChemicalMolecularStructuresthroughGaming
507

with (Hebenstreit, 1980), research in computer
assisted learning started in the early 1960’s in
several French Universities. Nevertheless, the
earliest references to its consistent use in Chemistry
are found in the UK, associated to the project
Computers in Teaching Initiative (Gagan, 2008), in
1989.
The number of e-learning products (or claimed
as such by its developers) has grown exponentially
since then (Casher et al., 1998), as illustrated also in
(Table 1). Note that the numbers in (Table 1) also
include items such as papers, reports, videos,
images, and other applications. The huge number of
web pages dedicated to Chemistry is an indication of
the relevance of information technologies in the
Chemistry learning process and corroborate the
statement that “educational technology is now
widespread in Chemistry teaching” (Gagan, 2008);
(Wodward, 2008). In fact, (Gagan, 2008) is a review
on the chemistry learning experience in the UK, so
its generalization to Europe or to the world should
be made with great caution. Nevertheless, if before
the Bologna Process
3
University Curricula across
Europe were very different, today the situation is
much more uniform. Moreover, we can see that their
conclusions are in line with the results shown in
Table 1.
Table 1: Quantity of results returned from a few Google
queries.
Google Query # Results
“e-learning in chemistry” 22 200
"Chemistry Virtual Lab" 22 200
"Chemistry Games" 116 000
+chemistry +e-learning 9 960 000
+Chemistry +"Virtual Lab" 1 110 000
+Chemistry +Game 70 000
2.2 Contemporary Web Solutions
After the global dissemination of web access, it is a
common practice to use standard platforms as a
support for e-leaning activities
4
. According to
(Kahiigi et al., 2008) these platforms may be
described as:
1. Learning Management Systems (LMS): a whole
range of information systems and processes that con-
tribute directly or indirectly for learning and for the
management of that learning. They are primarily
3
http://www.bologna-bergen2005.no/
4
According with http://moodle.org/sites/ there are 72105 active
sites registered in 223 countries.
developed to provide online learning services for
students, teachers, and administrators.
2. Content Management Systems (CMS):
developed to facilitate the collaborative creation of
contents, organisation, control, and to manage the
publication of documents in a centralized
environment.
3. Learning Content Management Systems
(LCMS): web-based systems that combine
management and administrative functionalities of
LMS and CMS for author approval, publishing, and
management of learning contents.
Being a recent classification that is used by a large
number of different research & development areas
and users, the concepts, acronyms and definitions
proposed by these authors should not be seen as a
final taxonomy. Just to give a practical example of
such differences, (Kahiigi et al., 2008) states that
moodle is an example of a CMS (and CMS stands
for Content MS) while moodle.org defines itself as
“a Course Management System (CMS), also known
as a Learning Management System (LMS) or a
Virtual Learning Environment (VLE)”.
2.2.1 Web Solutions for Chemistry
The number of web pages dedicated to Chemistry is
huge and includes many different applications
(Georgious et al., 2007), such as repositories of
theoretic materials, quizzes and questionnaires,
detailed descriptions of experiments, mechanisms of
chemical reactions, periodic tables, virtual
laboratories (Table 2), or games repositories (Table
3).
2.2.2 Virtual Laboratories
Considering the meaning of these two words:
1. Laboratory: a facility that provides controlled
conditions in which scientific research, experiments,
and measurement may be performed
5
.
2. Virtual: this term has been defined in philosophy
as "that which is not real" but may display the salient
qualities of reality. The use of the word virtual for
computer simulation of reality is not recent. The
Online Etymology Dictionary reports that the sense
of "not physically existing but made to appear by
software" appears as early as 1959
6
.
the concept of “Virtual Laboratory” should simply
qualify facilities that are not real but may display the
5
Source: http://en.wikipedia.org/wiki/Laboratory
6
Source: http://en.wikipedia.org/wiki/Virtual
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
508
salient qualities of the reality, to provide controlled
conditions in which scientific research, experiments,
and measurements may be performed.
However, as shown from the few examples
described in Table 2 and as already reported by
(Georgious et al., 2007), only a few “Virtual
Laboratories” actually allow virtual experiments. In
fact, the concept of Virtual Laboratory is used by the
Chemistry community for web sites that provide
different kinds of resources, ranging from theoretical
material, to questionnaires (that may be in a game
format or not), demonstrations of experiments or
even detailed experimental procedures.
Just to mention some of the selected sites, the
Portuguese “Laboratório Virtual de Química”
developed by Minho University is a depositary of
supporting information (materials & methods,
procedures, queries, etc.) to be used by the students
before their experimental lab classes. The Virtual
Chemistry Lab, from infoPlease, is a completely
different application, as it provides the possibility to
execute several experiments online, representing
what is occurring at the molecular level; this site can
be used both by students (to improve concepts) and
by teachers (to better illustrate these concepts to
their classes). Virtlab, that claims to have users
registered from 100 nations, can be seen as a good
tool to illustrate theoretical concepts (during a class,
for instance), or to compare with calculations results.
2.2.3 Virtual Reality Chemistry
Laboratories
Virtual Reality has to be considered as a valid option
for the integrating environment of the future Virtual
Laboratory. It exists already some Literature
concerning Virtual Reality applied to Chemistry,
ranging from overviews and tutorials about how to
develop such labs (Casher et al, 1998), to a set of
already implemented Virtual Reality solutions
(Georgiou et al, 2007); (Pérez et al., 2009).
Augmented Reality solutions (Azuma, 1997); (Braz
et al., 2007) are also worth to mention, as significant
progresses have recently been made in this field
(Núñez et al, 2007).
However, while a Virtual Reality environment is
seriously being considered to be used in the future
Virtual Lab, the use of an Augmented Reality
environment is out of the question, mainly because
of economic and technical reasons.
2.2.4 Chemistry Games
A simple Google query on Chemistry games gives
from 70E3 to 116E3 results (Table 1). These games
have, however, to be divided into games that really
require or illustrate some Chemistry, and others that
only use Chemistry as a theme or scenario, requiring
no real knowledge in the subject. In (Table 2) games
belonging to each category are presented. If we only
consider games that require an advanced level of
knowledge (Keck, 2000) we can state that most of
them are generally based on questionnaires, whether
a “time-attack” strategy is present or not.
Table 2: A few examples of claimed “Virtual
Laboratories”. Under the item “Demos” we include,
tutorials, theoretical material etc.
Name URL Chemistry Materials
Exper
iment
Quizz Demo
Virtual
Chemistry
http://www
.chem.ox.
ac.uk/vrch
emistry/
i)
Movies of reactions between salts and
reagents. ii) The reagents of an organic
reaction appear and the student has to
draw the curly rows to indicate the
mechanism of that reaction. The
application answers back, saying
whether it is correct or not and giving
tips in the second case. iii) VSEPR: The
program shows one molecule, from a
list of 20, and the user has to select its
molecular geometry from a list. In case
it is correct, it shows a 3D
representation of that molecule.
Yes Yes Yes
VLabs
http://vlabs
.uminho.pt
/quimica/q
uimica.htm
l
Presents a list of experimental works,
including material & methods,
proceedings, safety instructions and
movies showing the experimental
procedures or illustrating particular
techniques.
Yes
Lab Virtual
de
Química
http://www
2.fc.unesp
.br/lvq/me
nu.htm
Description of several experiments:
involved materials and procedures.
Yes
Virtual
Chemistry
Lab
http://www
.infoplease
.com/che
mistry/siml
ab/
Illustrates several kinds of reactions at
molecular level. It is very interesting to
illustrate the role of each molecule
during a chemical reaction in solution.
Yes Yes Yes
Virtlab
http://www
.virtlab.co
m/main.as
px
A
nimations of several experiments
showing the evolution of several data in
time (concentrations, pH, temperatures,
etc.)
Yes
Hi
hydrogen
http://libra
r
y.thinkque
st.org/114
30/researc
h/index.ht
ml
virtual experiments. The student virtually
“makes” the experiment and takes
conclusions.
Yes Yes
Electronic
Homework
Pages-
CSUDH
Chemistry
Dpt.
http://che
mistry2.cs
udh.edu/h
omework/
hwintro.ht
ml
Exercises to do at home. At the end the
student puts his name and the name of
his instructor. We believe each
instructor has access to his students’
scores. It is not a game. It is just one
more source of exercises to solve.
Yes
3 MAIN CONTRIBUTION
Our main contribution should be evaluated against
existing solutions:
1. Paper exercises
2. Web questionnaires
3. Existing solutions for VSEPR study
Considering our own experience with the two first
items and the validation results presented below on section
5, a few words should be said about the identified tutorial
LeMo-StudyingChemicalMolecularStructuresthroughGaming
509
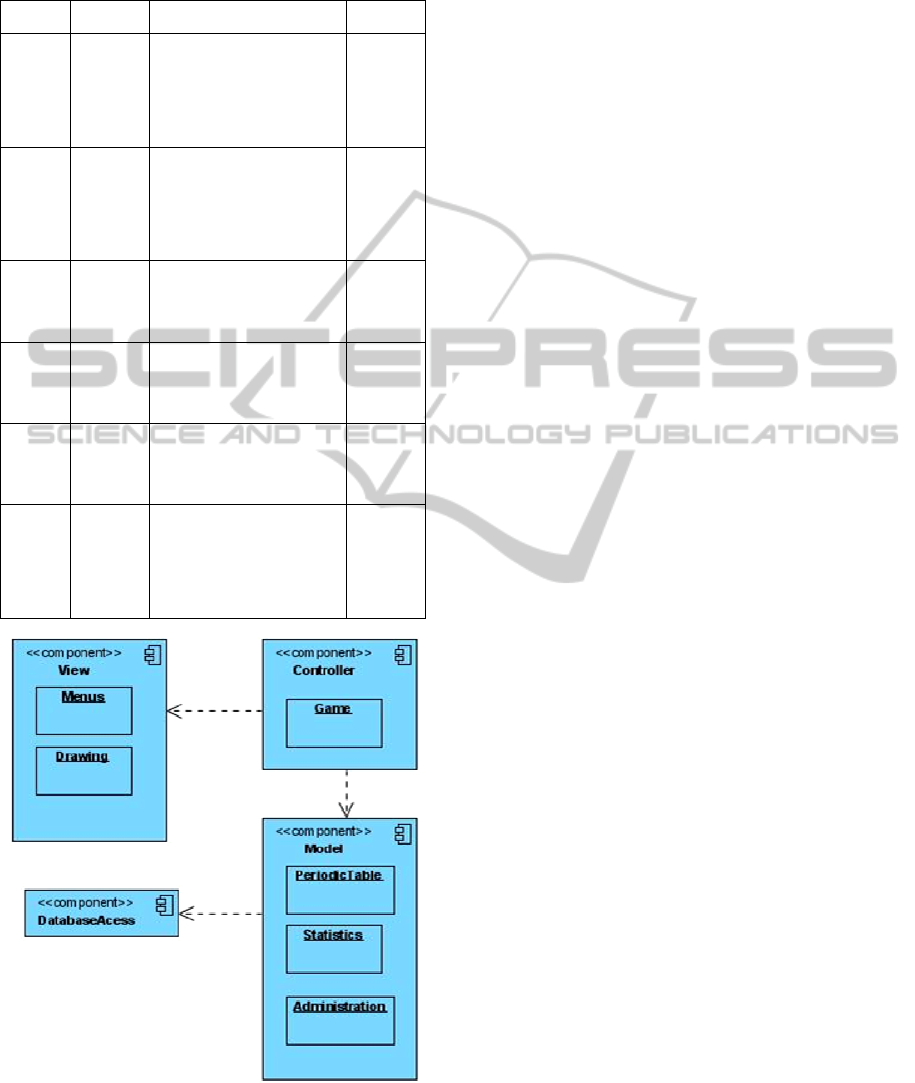
Table 3: Examples of web pages with Chemistry games.
PT stands for Periodic Table; CR stands for Chemical
Reactions.
Game URL Description
Chemistry
Level
Chemistry
Games
http://www.s
heppardsoft
ware.com/El
ementsgame
s.htm
Learning mode: the user clicks over
the symbol of an element and its
main properties and characteristics
are displayed;
Gaming mode: questions about
families, elements, symbols of the
PT elements.
From
elementary
to advanced
Penalty
Shootout
Games
http://www.c
anaryzoo.co
m/Chemistry
%20Games.
htm
1. The user chooses a goal keeper.
2. A quiz appears. If the answer is
right, the user may shootout a
penalty and, if he has luck, it’s goal.
At the final of the game, there is a
score of the number of right
answers and the number of goals.
Elementary
Games for
School
Students
http://www.rs
c.org/Educati
on/SchoolSt
udents/game
s.asp
A set of games and tutorials that
range from the PT through CR, oils,
etc.
School level
Creative
Chemistry
http://www.cr
eative-
chemistry.or
g.uk/
Set of applications covering
elementary chemical chapters.
Some time-attack questions,
“impossible mission quizzes”, and
Chemical crosswords.
School level
General
Chemistry
Jeopardy
Games
http://cheme
d.chem.pitt.e
du/Jeopardy/
genchem/ind
ex.htm
Jeopardy games covering several
chapters of a Chemistry University
level course.
University
level
Chemistry
Lab
Escape
http://www.pl
ayonlineflash
game.com/2
008/11/play-
chemistry-
lab-
escape.html
The player is trapped on a
chemistry lab and has to use the
available clues to escape. The user
doesn’t need to have large
Chemistry knowledge. It is more
important to pay attention and be
luck.
None
Figure 1: LeMo’s component model through a model-
view-controller architecture.
and quiz available at Virtual Chemistry (first row on
Table 1). Following a tutorial about VSEPR theory,
the program shows one from a list of 20 molecules
and the user has to select its molecular geometry
from a list. In case it is correct, the application pops
up a 3D representation of that molecule.
The present application (described below in
section 4) is completely different:
1. LeMo is a game: it has levels, scores, lives,
noises, other players, etc.
2. LeMo is more demanding in terms of Chemistry
contents: it also includes the writing of Lewis
formulas, the determination of the polarity of the
molecules, the selection of the right angles between
Chemical bounds, etc.
3. LeMo is a “student-friendly” application, as it
points out the mistakes in real-time, with pop up
messages whenever a mistake is made.
4. LeMo database is more complete. Presently
includes 50 different molecules / molecular ions and
the possibility of being extended is provided.
Actually, LeMo is a prototype of a new strategy for
learning through gaming, which we intent to validate
in order to continue the development of our own
Virtual Laboratory.
4 LeMo
4.1 LeMo’s Architecture
4.1.1 Component Model
LeMo’s architecture may be described through the
model architecture used to its development (Figure
1).
In the Model-View-Controller (MVC)
architecture we have identified three, or four
components, if we consider the database as
separated from the Model component, which
considering its relevance for the parameterization of
the whole game, we deed.
1. the “Model” package contains all the application
data, which responds to requests for information
about their status and responds to instructions to
change state;
2. the “View” package which gives the Model
classes a form suitable for interaction, for example,
placing the atoms in the periodic table so that the
user can interact with them;
3. The “Controller” package which receives data
input from the user, such as clicking a button that
initiates a response by making calls to objects of the
package Model.
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
510
4. Finally, the database component comprises three
main sets of information:
a. information about registered users;
b. the information about gaming and usage
statistics and, finally,
c. the information about the molecules and its
structure that are available for gaming.
This used architecture already proved its robustness
against changes in two ways: it took 4 person-hour
to completely translate the initial Portuguese
application used at EST Setúbal
(http://193.137.47.29/) to the English version
(http://193.137.47.29/ingles/) used in this paper and
the inclusion of a back office is being
straightforward, mainly due to the fact that it only
interacts with the database.
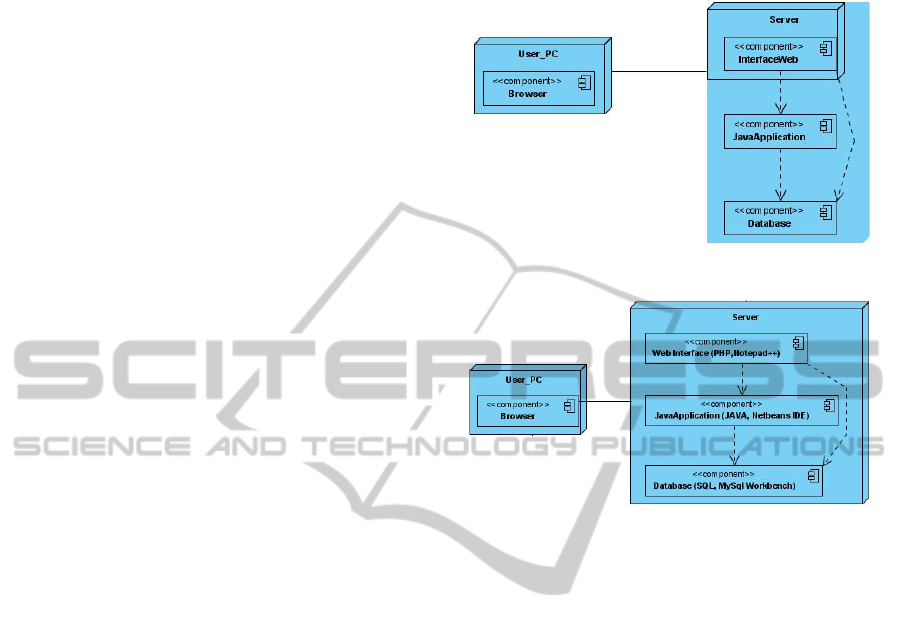
4.1.2 Physical Architecture
The physical architecture is pure client-server
(Figure 2), and the MVC component architecture
may be directly mapped to it: the View, Model,
Controller and database components are server
residents. The View component is a Web Interface
accessed by the client (user) computer through a
web browser and running on top of a web server.
The Model and Controller components are Java
Applications running on the server.
4.1.3 Development Platform
The database used was created with mysql. The web
interface is in php, so that users can alter their
profile if needed or simply play the game in the page
where it is called.
In selecting the technologies to be used, open
source technologies were preferred, not only because
they avoid unnecessary costs, but also because they
were more familiar. Java was chosen as the
programming language for the game application, as
it is present in most operating systems used around
the world, is used by both computers and mobile
devices, its robust, it has a very active community,
among other factors. In order to have the application
on the Web, an applet was decided to be created.
The development environment picked was
NetBeans IDE, as we consider IDE with a graphical
environment pleasant and easy to use.
To develop the application site, php was chosen
as language, as it is very powerful, very productive,
easy to learn and well suited for the development of
Web applications. Php is also easy to connect to the
database, becoming ideal for this application. The
choice of mysql as the DBMS is due to its
performance, and reliability.
Figure 2: LeMo’s Physical Architecture.
Figure 3: Development platform.
4.2 Playing LeMo
4.2.1 Access and Register
The first thing to do to experiment LeMo is going to
http://193.137.47.29/ingles/.
1. If it is the user’s first time, he should register.
2. After registration is complete, the user will be
redirected to the contacts page where a welcome
message appears and the player has access to his
email address (in case there is any problem or the
user wishes to give any suggestion).
4.2.2 Main Menu Options
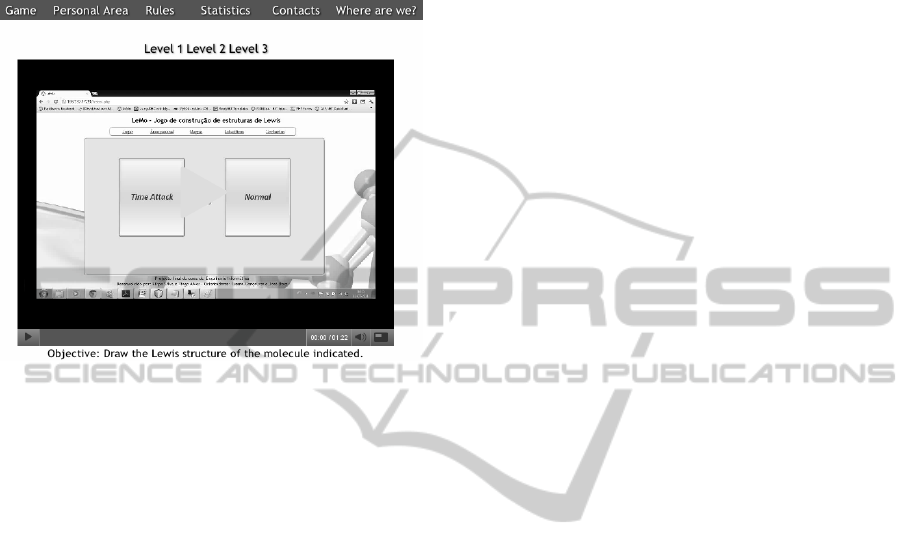
Looking at the top menu on Figure 4 there are six
options: “Game”, “Personal Area”, “Rules”,
“Statistics”, “Contacts” and “Where we are?”
3. Selecting “Where we are?” the user’s location
will be presented in Google Maps.
4. If “Statistics” is selected, four tables of
punctuation will appear: maximum, minimum,
medium and stored. This tables show the top ten
players in each category.
5. Selecting “Rules”, the user has the possibility of
watching 3 videos showing how to play. Each video
LeMo-StudyingChemicalMolecularStructuresthroughGaming
511
is a demonstration of each level of the game. Bellow
the video, the objective of each level is described.
6. Selecting “Personal Area” the user has the
possibility to change all his personal data, except the
email address.
Figure 4: Rules page.
4.2.3 Playing the Game
Finally selecting “Game” the player will have access
to game itself. When the game starts the player has
to choose between two modes of gaming, normal or
time attack (Figure 4).
The difference between them is that in time
attack mode there is a clock running backwards that
ends the game when the timer gets zero, sending an
alarm sound. The game also ends when the number
of lives equals to zero in both playing modes.
If the normal mode is selected the look of the
game will be has presented in Figure 5.
At the first level the player is supposed to draw
the Lewis formula of a given molecule or molecular
ion. In the example illustrated in Figure 5 the
chemical compound assign is CN
-
. At the left side
the user can find the total number of lives, the actual
number and his score. At the top of the game area
there is a periodic table and at the right side there are
four buttons, with the three types of possible bounds
to be formed between atoms (single, double and
triple bounds) and a lone pair of electrons not shared
(which is also a possibility for these formulas).
The player has to drag and drop the atoms from
the periodic table and the bounds/not shared electron
pairs form the connection buttons to the drawing
area in order to draw the Lewis formula of the
assigned molecule/molecular ion.
These elements can be rotated and deleted from
the drawing area by clicking with the right mouse
button; the atom element can also be given a positive
or negative formal charge using the same button.
If the structure to be drawn is a resonance hybrid
(which means that has to be represented by several
Lewis formulas, each one representing each
contributing structure) the player has to choose the
resonance hybrid option, above the drawing area and
select how many contributing structures are necessary.
Several extra drawing areas will be created, depending on
the number of contributing structures. When the player
finishes the Lewis formula representation, he clicks the
confirm button.
If the Lewis formula is correct, score will be
added to the score board and a congratulations
message will appear, also asking whether the player
wants to proceed to the next level or quit.
In case the structure is wrong, score will be
removed from the score board, a life will be taken
and a message mocking with the player and given
tips about the possible mistakes he made will appear.
After the user succeeds in the first level and
confirms the wish to continue, the second level
layout appears. In this second level (Figure 6) the
player has to attribute geometry to the
molecule/molecular ion which Lewis formula he
drawn in the previous level, using the VSEPR
theory.
At the top of the game area there are two
dropdown boxes that the player will use to select the
correct combination of angle and geometry.
Whenever the player chooses a possible
combination an animated 3D image of the geometry
appears. Note that this only indicates that the
combination is possible, not that it is correct.
After choosing the combination the player has to
click in the confirm button. Then, like in the first
level, in case the player has succeed, a
congratulations message will appear, also asking
whether the player wants to continue to the third and
final level. Score will also be added to the score
board. In case the player chosen a wrong geometry,
score will be removed from the score board, a life
will be taken and a message mocking with the player
will be shown.
Once the player has completed the second level
and confirmed he wants to continue, the third and
last level layout is shown.
In this third level, as shown in Figure 7, the atoms
are presented in the same locations the player has
chosen at the first level (which may not correspond
to their real locations, according to the VSEPR
theory and the attributed geometry).
The player has to drag and drop the vectors to the
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
512
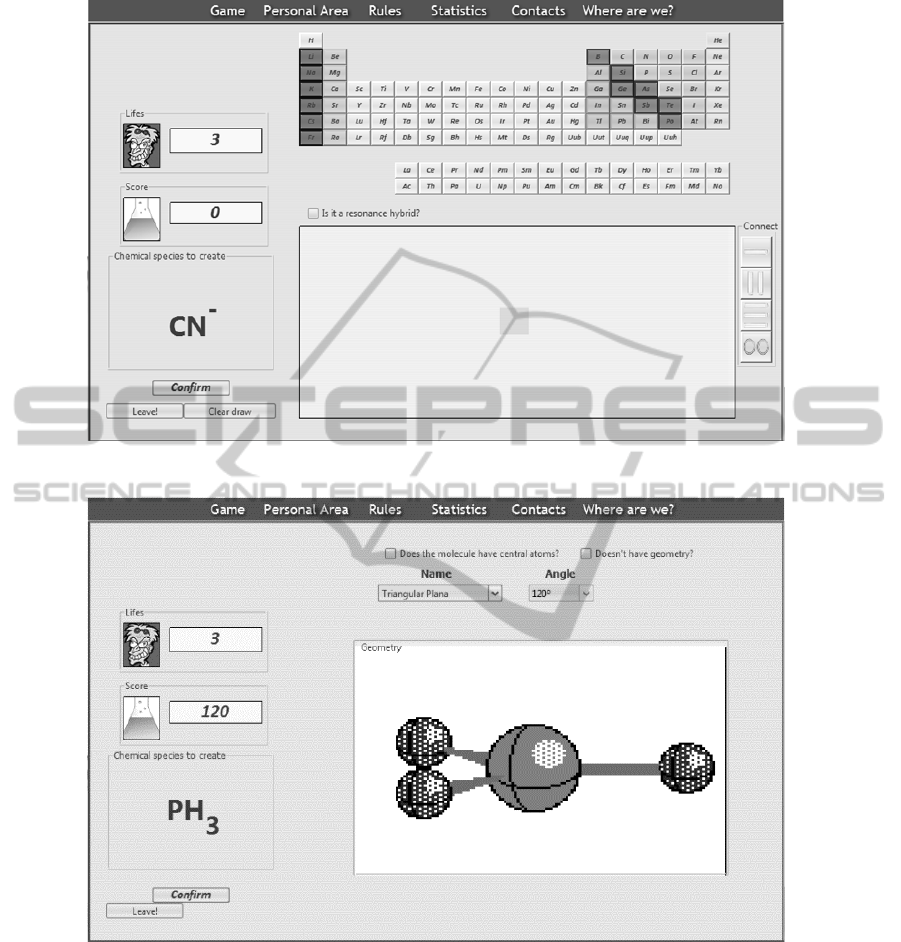
Figure 5: First level in normal mode.
Figure 6: Second level in normal mode.
draw area in the right place and direction, and select
the polarity of the structure in a dropdown box for
that purpose.
If the player succeeds, the game is over. Score
will be added to the score board and a congratulation
message with his archived rank is shown at the
sound of fireworks. At the same time the total score
of the player is transferred to the database, to be
used in the statistics of the game.
The player may then choose to play again in
order to get a higher score or to leave the game.
4.2.4 Funny Error Messages and
Competition
All messages in this game have a type of language
oriented to the students.
Since the main objective of the game is to be fun
in order to increase the study duration and improve
students memory in this topics, all messages have
LeMo-StudyingChemicalMolecularStructuresthroughGaming
513
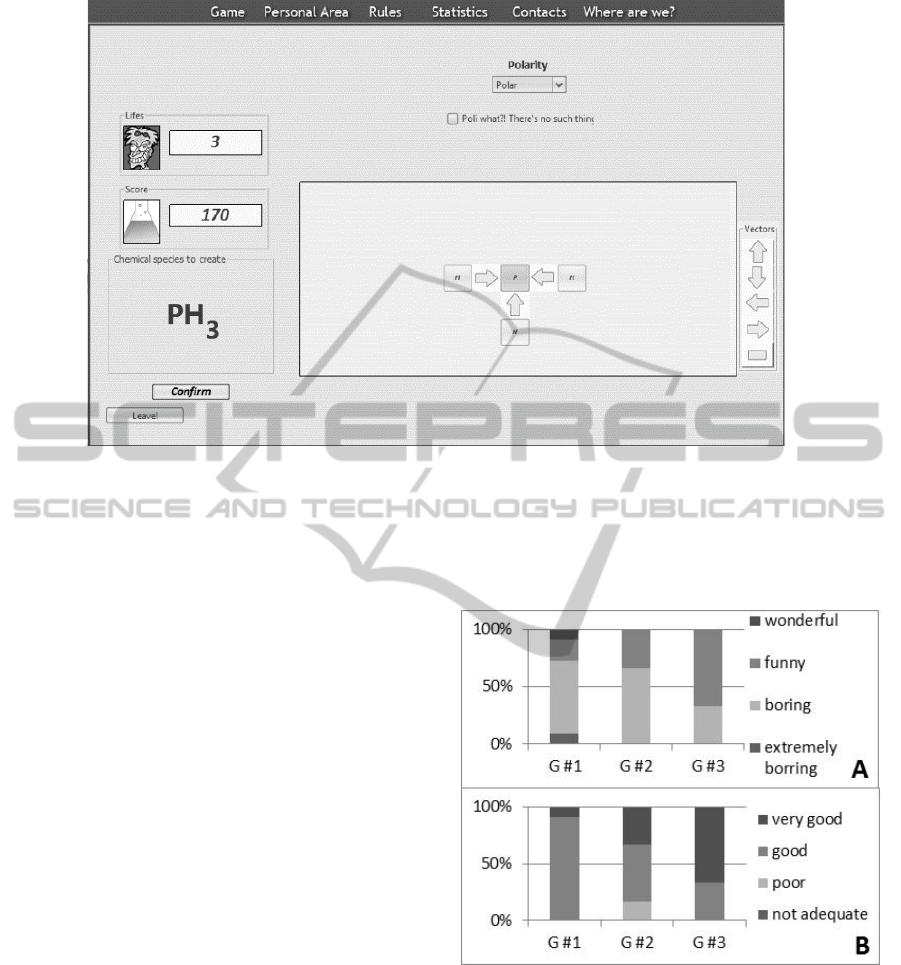
Figure 7: Third level in normal mode.
also funny sounds and the game has music in
background (that can, however, be turned off).
In order to increase competition between
students and increase the time of study, the players
may consult the high scores, accumulated scores,
minimum scores and medium score charts.
5 VA L I D AT I O N
A group of 30 students attending the Chemistry
course of the 1
st
year of Mechanical Engineering in
EST Setúbal were divided into three test groups.
During one week group #1 was only allowed to
study by the exercise book, group #2 by the moodle
interactive exercises and group #3 by LeMo. All
these students were attending the Chemistry course
for the first time. After one week, all students were
submitted to a written test and an inquiry.
As seen in (Figure 8), 64% of the students using
the exercise book found that method boring and 9%
very boring. 67% of the students using moodle still
found it boring, but as much as 33% answered that it
was fun. In the test group #3, only 33% of the
students classified it as boring. Indeed, 67% of the
students said that studying by LeMo was a funny
alternative.
The same figure also shows that 91% of the
students using the exercise book considered it as a
good method and 9% as very good. The opinions
were more spread in the group of students that
solved the moodle virtual exercises: 17% classified
the method as poor, 50% as good and 33% as very
good. The majority of the students from group #3
(67%) considered LeMo a very good method for
studying molecular structures and 33% good.
Figure 8: Answers of the students from each of the three
groups to the same question “How do you classify the
method you used to study this chapter?”.
For 90% of the students in group #1, the major
advantage of the exercise book is that they are used
to it. For group #2, 67% of the students reported that
the major advantage of solving the interactive
exercises on moodle is the pop up tips that appear
whenever students give wrong answers. Concerning
LeMo, students were required to point out the three
main advantages: 70% mentioned the real-time error
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
514

messages, 40% referred the competition with the
colleagues and 40% stated the game was user-
friendly. Note that moodle tips are much more
generalist than the error messages of LeMo.
LeMo was well accepted by students, which
identified and appreciated the fun character of the
game and considered it a good tool for studying
these matters.
Normal mode is preferred by 83% of the
students, as it allows more time to think and to read
the messages, while 17% prefer time-attack mode,
saying that it is more challenging.
6 CONCLUSIONS AND FURTHER
WORK
As said above, the aim of LeMo is to provide a new
method for practicing the writing of Lewis formulas
and applying VSEPR theory in a game format, in
order to both motivate students not to give up and to
help them memorizing images and concepts though
emotions.
The developed application is absolutely new and,
to our knowledge, completely different from all the
others found in the cyberspace. It is more complete
in terms of contents, has a larger database, is funnier
than other applications dedicated to Chemical
Structures, and is more adequate then other previous
attempts made by the same group, as the moodle
inquiries.
The evaluation of LeMo shows that a large
majority of the students preferred to study using this
new application. They considered LeMo a very good
tool mainly because of the real-time pop up
messages that point out their mistakes but also
because they found it funny and challenging, which
induces positive emotions. Are the latest
contributing to their learning process? It is our
opinion that further work is still required. Further
validation tests are already planned to occur during
the final exam of the Chemistry course for
Mechanical Engineering students, with a larger test
group (≈ 130 students) and with a new enquire
(more directed to emotions).
As said, LeMo was developed as a permanent
beta release, to be incrementally updated according
to the users’ feedbacks. Three tasks are already
scheduled:
1. Development of a back-office allowing an easier
introduction of new molecules in the database,
replacing the current process (that involves tables) –
this task is already in progress.
2. The full integration into the EST Setúbal e-
learning platform, rather than the current link to the
LeMo hosting site.
3. Development of an integrating environment,
possibly using Virtual Reality, where LeMo will be
one of the available applications.
Tasks number 2 and 3 are planned to start as soon as
the second validation process is finished and gaming
success unequivocally proven. They will mark the
beginning of the ESTSetúbal Chemistry Virtual
Gaming-Lab.
REFERENCES
Allen, E. And Seaman, J.; 2008; Staying the Course:
Online Education in the United States, 2008, Sloan C..
Allen, E. And Seaman, J.; 2010; Class Differences: Online
Education in the United States, 2010. Sloan C..
Azuma, R.: A Survey of Augmented Reality, in Presence:
Teleoperators and Virtual Environments, Vol. 6, nº4,
pp. 355-385, 1997.
Braz, J. and Pereira, J. ; 2007; TARCAST: Taxonomy for
Augmented Reality CASTing with Web Support; The
International Journal of Virtual Reality, 2008,
7(4):47-56
Casher, O., Leach, C., Page
,
C. And Rzepa H.; 1998;
“Virtual Reality Modelling Language (VRML) in
Chemistry”; Chemistry in Britain, 1998, vol. 34, p. 26.
Bottentuit, B. and Coutinho, P.; 2006; “Laboratories
Based on Internet: comparative analysis of current
experiences and development of a virtual laboratory”
in Méndez-Vilas, A. [et al.], ed. lit. – “Current
developments in technology-assisted education:
proceedings of the MICTE, 4, Seville, Spain, 2006”.
Badajoz: Formatex, 2006. vol. 7, p. 1284-1289. ISBN:
84-690-2469-8
Damásio, A., 1994. Descartes' Error: Emotion, Reason,
and the Human Brain, Putnam, 1994; revised Penguin
edition, 2005.
Damásio, A., Damasio, H., 1999. Self Comes To Mind -
The Evolution of Consciousness, Cornerstone, ISBN:
9780434015436
Damásio, A., 2010. Self Comes to Mind: Constructing the
Conscious Brain, Pantheon, 2010.
Espinilla, M., Palomares, I., Bustince, H.;2010; “Design
and Development of On-line Educational Games
Based on Questions”; CSEDU 2010 - 2nd
International Conference on Computer Supported
Education; 6-8 May 2011, Noordwijkerhout, The
Netherlands.
Gagan, M.; 2009; “Review of the Student Learning
Experience in: Chemistry”; The Higher Education
Academy Physical Sciences Centre; May 2009; ISBN
978-1-903815-25-0
Georgiou J., Dimitropoulos K., and Manitsaris A.; 2007;
“A Virtual Reality Laboratory for Distance Education
LeMo-StudyingChemicalMolecularStructuresthroughGaming
515

in Chemistry”. International Journal of Social and
Human Sciences 1, p 306-313.
Hebenstreit, J.; 1980; “Computer-Assisted Instruction in
France: present situation and prospects for the future”.
Swail, E. and Neal, G. (Eds.) Proceedings of the Third
Canadian Symposium on Instructional Technology,
Vancouver, February 27, 1980, Ottawa: National
Research Council Canada, pp. 77-91.
Kahiigi, E. K. , Ekenberg, L.,Hansson, H., Tusubira, H.
and Danielson, M., 2008. “Exploring the e-Learning
State of Art.” The Electronic Journal of e-Learning
Volume 6 Issue 2, pp77 – 88.
Keck, M. V., 2000; “A Final Exam Review Activity Based
on the Jeopardy Format”; J. Chem. Educ. Volume 77
pp-483.
Núñez, M., Quirós, R., Carda, J. and Camahort, E.; 2008;
Collaborative augmented reality for inorganic
chemistry education. In Proceedings of the 5th
WSEAS/IASME international conference on
Engineering education (EE'08), Mauri, J., Zaharim,
A., Kolyshkin, A., Hatziprokopiou, M., Lazakidou, A.,
Kalogiannakis, M., Siassiakos, K. and Bardis, M.
(Eds.). World Scientific and Engineering Academy and
Society (WSEAS), Stevens Point, Wisconsin, USA,
271-277.
Pérez, Z.and Cox, R.; 2009; “Teaching safety precautions
in a laboratory DVE: the effects of information
location and interactivity”; Computación y Sistemas
Vol. 13 No.1, 2009, pp 96-110; ISSN 1405-5546.
Rekkedal, T. and Qvist-Eriksen, S. 2003. Internet Based
E-learning, Pedagogy and Support Systems, in: H.
Fritsch (Ed) The role of student support services in e-
learning (Hagen, FernUniversitat ZIFF Papiere 121)
Shacham, M. and M. B. Cutlip, "Educational Utilization of
PLATO in Chemical Reaction Engineering." Comp. &
Chem. Eng., Vol. 2, 197 (1981).
Shen, L., Wang, M., and Shen, R. ,2009. Affective e-
Learning: Using “Emotional” Data to Improve
Learning in Pervasive Learning Environment.
Educational Technology & Society, 12 (2), 176–189.
Smith, S.; 1970. “The use of computers in the teaching of
organic chemistry”. J. Chem. Educ., 1970,47(9), p
608. DOI:10.1021/ed047p608
Tavangarian, D., Markus, E., Nölting, L., Nölting, K.,
Röser, M., 2004. “Is e-Learning the Solution for
Individual Learning”. Electronic Journal of e-
Learning, Vol. 2, No. 2. (December 2004)
Yoshimura, T., 2006. J. Comput. Chem. Jpn., Vol. 5, No.
3, pp. 129–138 (2006) Development and
Popularization of E-Learning Chemistry Educational
Resources in Japan
CSEDU2012-4thInternationalConferenceonComputerSupportedEducation
516
