
Recovery of Sequential and Non Sequential Memories with a Neural
Mass Model
Filippo Cona and Mauro Ursino
Department of Electronics, Computer Sciences and Systems, University of Bologna, Via Venezia 52, Cesena, Italy
Keywords: Neural Mass Models, Memory, Neural Assemblies, Autoassociative Networks.
Abstract: A neural model for the recovery of learnt patterns is presented. The model simulates the theta-gamma
activity associated to memory recall. Two versions of the model are described: the first can learn generic
patterns without a given order, while the second learns patterns in a specific sequence. The latter has been
implemented to overcome the limited recovery capacity of the former. The network is trained using Hebbian
and anti-Hebbian paradigms, and exploits excitatory and inhibitory mutual synapses. The results show that
autoassociative memories for storage and recovery of multiple patterns can be built using biologically
inspired models which simulate brain rhythms, and that the model which learns sequences can recover much
more patterns.
1 INTRODUCTION
The execution of complex tasks requires the
integration of many different pieces of information
spread across the whole brain. A widely used
concept to describe the large-scale integration in the
brain is the Neural Assembly (NA), which is defined
as the ensemble of neurons that take part in the
realization of a single cognitive task.
A popular hypothesis, named “binding by
synchronization” (Varela et al., 2001); (Singer,
1999), suggests that the formation of NAs is
obtained through the phase synchronization of
neural groups in the gamma band (>25Hz).
According to this hypothesis, a NA can be formed
rapidly and can rapidly be disrupted under the
influence of external or internal events to make
place for alternative NAs. Furthermore, the recent
observation that gamma rhythms are modulated by
slower theta rhythms (4-7Hz) suggests the idea that
the construction and degradation of gamma rhythms
is under control of theta oscillations (Canolty et al.,
2006); (Doesburg et al., 2009).
Several mathematical models in past years
described the formation and segmentation of NAs,
simulating how the brain can assign a neural group
to the correct assembly, while maintaining it
separated from neural groups which belong to other
assemblies. However, these models used very simple
oscillating neural units, such as Wilson Cowan or
relaxations oscillators (Wang et al., 1990); (von der
Malsburg and Buhmann, 1992); (Wang and Terman,
1997); (Ursino et al., 2003); (Ursino et al., 2009),
which are unable to simulate realistic brain rhythms.
A recent work addresses the same issue using a more
sophisticate model, able to mimic the electrical
activity in cortical regions (Cona et al., 2012). Using
a mathematical model for the simulation of brain
activity, known as neural mass model (NMM), the
authors proposed a neural architecture that can learn
different NAs and evoke them separated in time.
However, they were able to evoke just three gamma
oscillations within each theta cycle, while many
works suggest that this number can be between 4
and 7 (Cowan, 2000); (Miller, 1956).
The model itself suggested how to overcome this
limitation: by visual inspection of the simulated
waveforms, one can observe that most of the time
between two consecutive gamma oscillations is
‘wasted’ to determine which NA should follow. So
we inferred that, if patterns are learnt in a specific
sequence, the model should be able to evoke much
more NAs within a single theta cycle. This
corresponds to psychophysical observations.
In the following, we will show the architecture of
the NMM used to memorize non-sequential NAs and
the changes adopted to learn a sequence of NAs. The
different performance of the two models will be
outlined.
547
Cona F. and Ursino M..
Recovery of Sequential and Non Sequential Memories with a Neural Mass Model.
DOI: 10.5220/0004154005470551
In Proceedings of the 4th International Joint Conference on Computational Intelligence (NCTA-2012), pages 547-551
ISBN: 978-989-8565-33-4
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
2 METHOD
2.1 The Cortical Column
The fundamental unit of our network is the model of
a cortical column. It consists of four interconnected
neural populations (Figure 1), which are pyramidal
cells, excitatory interneurons and inhibitory
interneurons with slow and fast kinetics
(respectively referred to as GABA
A,slow
and
GABA
A,fast
). The interaction of these populations
allows the cortical column to generate different
oscillatory activities, while the output of the model
(the simulated EEG signal) is given by the
membrane potential of the pyramidal cells. See
Ursino et al., (2010) for more details on the model.
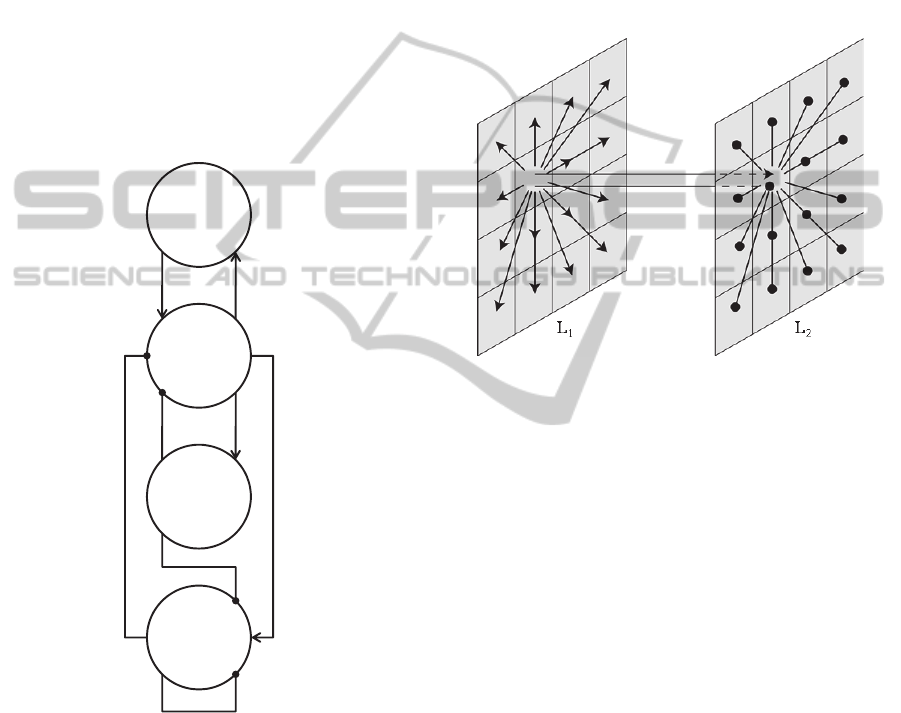
Figure 1: Connectivity between neural populations in the
model of a cortical region.
2.2 Recall of Non-sequential Patterns
The neural network consists of 2 layers (L
1
and L
2
)
of M×N cortical columns each, (Figure 2). The first
receives the external input and sends its output to the
second layer. The second generates the cortical
output. The network can learn patterns (NAs) of
features. Each feature corresponds to a position in
both layers and therefore is associated with two
oscillatory columns, one in L
1
and one in L
2
.
Each unit in L
1
and L
2
is represented through a
cortical column, according to the diagram in Figure
1. Moreover, units in the same layer communicate
through lateral synapses, according to the schematic
representation in Figure 3. These synapses were
learnt via Hebbian or anti-Hebbian mechanisms,
during a training (storage phase) in which seven
complete patterns (without any lacking feature) were
given separately to the network. After training, the
network was tested using incomplete patterns as
inputs (recovery phase).
Figure 2: Architecture of the two layers: arrows indicate
connections toward pyramidal cells, while circles indicate
connections toward GABA
A,fast
interneurons.
During the recovery phase L
1
works as an
autoassociative memory, i.e., it serves the main
function of recovering the lacking information when
the patterns are not totally stimulated. Hence, just a
few features are sufficient to evoke the whole NA
they belong to. The units in L
1
exhibit an oscillatory
activity in the theta band which drives L
2
. This
behaviour is obtained by training the excitatory
lateral connections in L
1
with the Hebb rule, i.e.,
reinforcing excitation among features which belong
to the same pattern and so are simultaneously active.
The excitation between features belonging to the
same NA ensures the complete recovery of lacking
information. However, excitation would lead
neurons to the upper saturation (i.e., to a maximal
excitation). The theta oscillation emerges thanks to
the presence of inhibitory populations with slow
synaptic kinetics (GABA
A,slow
interneurons) which
progressively inhibit units through synapses with a
time constant of 34 ms.
L
2
plays two roles: it makes the cortical columns
oscillate synchronously in the gamma band when
they encode for the same NA (binding), and out-of-
synch if they belong to different NAs
Excitatory
interneurons
Pyramidal
cells
Inhibitory
interneurons
(slow)
Inhibitory
interneurons
(fast)
IJCCI2012-InternationalJointConferenceonComputationalIntelligence
548
(segmentation). The synchronicity is obtained
through lateral inhibitory synapses, trained with the
Hebb rule which link pyramidal neurons in the pre-
synaptic units to GABA
A,fast
interneurons in all units
which encode for the same NA(Figure 3). As
demonstrated in previous works (Bartos et al.,
2002); (Bartos et al., 2007); (Ursino et al., 2010),
reciprocal fast inhibition is a powerful mechanism to
ensure synchronization of gamma rhythms. As a
consequence of this mechanisms, all units in the
same pattern become part of a bigger gamma
oscillator. The segmentation is obtained by training
another set of synapses from pyramidal to
GABA
A,fast
interneurons with an anti-Hebbian rule
(i.e., a synapse is reinforced when the pre-synaptic
unit is active, but the post-synaptic unit is silent).
The latter synapses, however, have a much faster
dynamics compared with the previous ones (rising
time less than 1 ms). These can simulate ultra-fast
AMPA synapses. This mechanism induces a very
fast inhibition between columns in different patterns
of L
2
, so that different features in different patterns
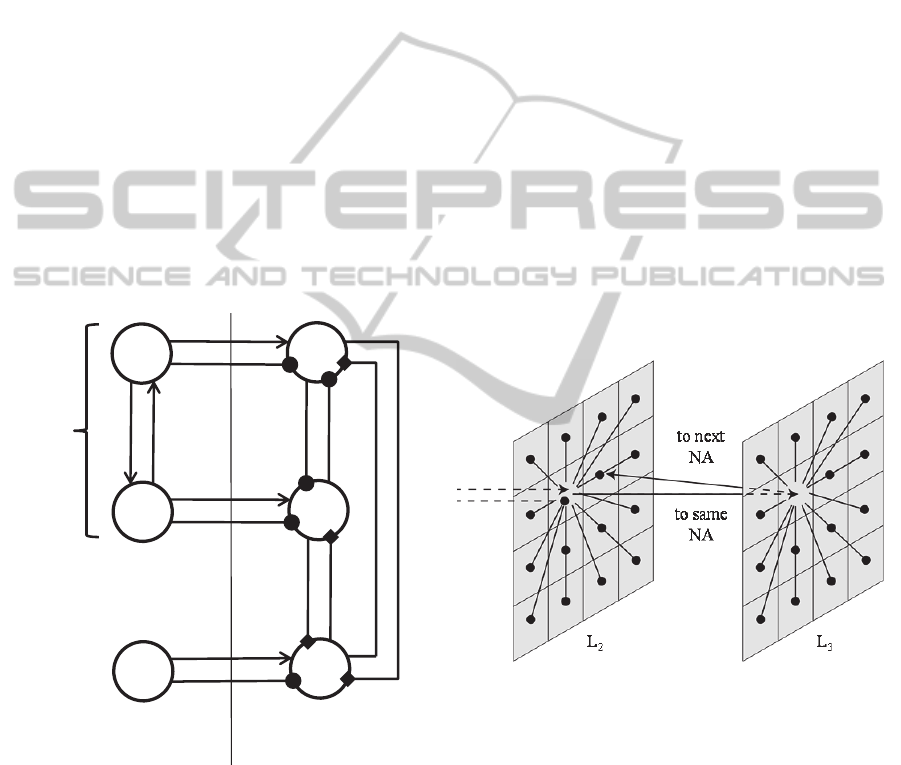
tend to desynchronise (Cona et al., 2012).
Figure 3: Connections between 6 regions, 3 in L
1
and 3 in
L
2
. The 4 upper regions encode for features belonging to
the same NA, while the lower 2 encode for a feature
belonging to another NA. Arrows, circles and squares
represent respectively pyramidal-pyramidal connections,
pyramidal-GABA
A,fast
connections and fast AMPA-
mediated pyramidal-GABA
A,fast
connections.
In conclusion, ultrafast anti-Hebbian synapses
from pyramidal to GABA
A,fast
neurons ensures a
very rapid desynchronization of units, while slower
Hebbian synapses from pyramidal to GABA
A,fast
neurons ensure synchronization in the gamma range.
2.3 Recall of a Sequence of Patterns
To recall patterns in a specified sequence we added a
third layer (L
3
) whose cortical columns are
connected in the same way as columns in L
2
. Units
in L
2
target units in L
3
which belong to the same
NA. On the other hand, units in L
3
target units in L
2
which belong to the next NA in the sequence (Figure
4). The connections between L
2
and L
3
are mediated
by the same synapses involved in the generation of
the gamma rhythm. These are learnt with the Hebb
rule during a training period, in which the different
patterns in a sequence are given to the network in
the correct order, delayed by 25 ms each.
Subsequently, the network is tested by providing just
the first pattern of the sequence as an input to L
1
.
Thanks to the trained synapses, when a NA pops out
in L
2
it pops out also in L
3
with a half gamma cycle
delay. In turn, a NA in L
3
evokes the next one in L
2
with the same delay. As a consequence, if the first
NA of the sequence is evoked in L
2
, all the other
NAs will follow with a gamma period delay each.
Figure 4: Architecture of the third layer and its
connections with L
2
: as in figure 2, arrows indicate
connections toward pyramidal cells, while circles indicate
connections toward GABA
A,fast
interneurons.
3 RESULTS
Figure 5 shows the behaviour of a NMM that has
learnt 7 simultaneous non sequential patterns. Note
that in L
1
all the NAs are activated and oscillate in
phase in the theta frequency range (4Hz in this case).
In L
2
the NAs are activated one at a time, but in each
theta cycle just a few (2 or 3) gamma oscillations
take place. Panel c shows that, when a NA
Same
NA
Other
NA
L
1
L
2
RecoveryofSequentialandNonSequentialMemorieswithaNeuralMassModel
549

completes its gamma oscillation (and thus stops
inhibiting other NAs) all the other ones compete to
start the new cycle. However, due to their mutual
inhibition, none of them prevails immediately and it
takes almost 50 ms for a new pattern to win the
competition. This delay prevents the system from
evoking more than 2-3 oscillations within a theta
cycle. All seven patterns can be recovered, but in
different theta cycles.
This observation suggests that, if at the end of a
gamma impulse one of the patterns is privileged
with respect to the other ones, the delays between
the oscillations in L
2
can be avoided, thus allowing
for the appearance of a greater number of gamma
impulses within a theta cycle.
Figure 5: Activity of 7 non-sequential patterns. Panels a
and b show the activity of 7 cortical columns (one for each
learnt pattern) in L
1
and L
2
respectively. Panel c shows a
zoom of the area within the dotted boundary in panel b.
Figure 6 shows that the modified network, which
learns a sequence of patterns in a well-established
order, can recover all 7 NAs within a theta cycle, in
response to the first pattern given as input. This is
due to the fact that at each gamma period one NA in
L
2
receives an additional excitation from the
previous NA in L
3
, thus greatly unbalancing the
competition between the different NAs to emerge.
4 CONCLUSIONS
We can draw some considerations on the results
obtained. The main point is that this modelling study
suggests a possible neural basis for an everyday
phenomenon: it is much easier to keep in mind
objects or concepts assigned in a given order, than
totally uncorrelated ones. Furthermore, the two
models can afford two alternative problems: i) to
store and recover different patterns provided
simultaneously; ii) to store and recover a sequence
of patterns, so that the first can evoke all the others
in the assigned order.
Figure 6: Activity of 7 patterns in a sequence. Panels a, b
and d show the activity of 7 cortical columns (one for each
learnt pattern) in L
1
, L
2
and L
3
respectively. Panels c and e
show zooms of the areas within the dotted boundaries in
panels b and d respectively.
Furthermore, the present model emphasizes the
strict relationship existing between theta and gamma
rhythms in memory storage and recall, a behaviour
which has been observed in many memory tasks,
such as during phase precession (Lisman, 2005) and
the formation of memories (Bikbaev and Manahan-
Vaughan, 2008) in the hippocampus.
A point which requires a brief discussion is the
learning capacity of the network. In this work we
0 0.5 1
0
1
2
3
4
5
Patterns in
L
1
firing rate (Hz)
0 0.5 1
0
1
2
3
4
5
Patterns in L
2
time (s)
firing rate (Hz)
0.1 0.15 0.2 0.25
0
0.5
1
Patterns in L
2
(detail)
time (s)
Column in pattern 1
Column in pattern 2
Column in pattern 3
Column in pattern 4
Column in pattern 5
Column in pattern 6
Column in pattern 7
a
cb
0 0.5 1
0
1
2
3
4
5
Patterns in L
1
firing rate (Hz)
0 0.5 1
0
1
2
3
4
5
Patterns in L
2
firing rate (Hz)
0.1 0.15 0.2 0.25
0
1
2
3
4
5
Patterns in L
2
(detail)
Column in pattern 1
Column in pattern 2
Column in pattern 3
Column in pattern 4
Column in pattern 5
Column in pattern 6
Column in pattern 7
0 0.5 1
0
1
2
3
4
5
Patterns in L
3
time
(
s
)
firing rate (Hz)
0.1 0.15 0.2 0.25
0
1
2
3
4
5
Patterns in L
3
(detail)
time
(
s
)
a
c
de
b
IJCCI2012-InternationalJointConferenceonComputationalIntelligence
550

trained the network with just 7 NAs, but there is no
theoretical limit to the number of NAs that can be
learnt, as long as there are enough cortical columns
to represent all features and the NAs are orthogonal.
The analysis of the network performance with non-
orthogonal NAs (i.e., with shared features) and the
comparison with other neural models for data
storage and recovery will be the subject of future
works.
A problem related to learning a longer sequence
is that the recall of the whole sequence in L
2
would
last more than one theta cycle; hence the following
theta cycle would begin in the midst of the previous
sequence. In this case a reset mechanism is required:
this should be realized to inhibit columns in L
2
and
stop the sequence when the theta cycle ends. It is
possible that dopamine plays a role in this process.
This study underlines the great value of
mathematical models as hypothesis generators, since
they allow exploration of all the mechanisms
involved, even those that are practically inaccessible
with a purely experimental approach.
REFERENCES
Bartos, M., Vida, I., Frotscher, M., Meyer, A., Monyer,
H., Geiger, J. R. P. and Jonas, P., (2002). Fast synaptic
inhibition promotes synchronized gamma oscillations
in hippocampal interneuron networks. Proceedings of
the National Academy of Sciences of the United States
of America, 99(20), 13222–13227.
Bartos, M., Vida, I. and Jonas, P., (2007). Synaptic
mechanisms of synchronized gamma oscillations in
inhibitory interneuron networks. Nature Reviews
Neuroscience, 8(1), 45–56.
Bikbaev, A. and Manahan-Vaughan, D., (2008).
Relationship of hippocampal theta and gamma
oscillations to potentiation of synaptic transmission.
Frontiers in Neuroscience, 2(1), 56–63.
Canolty, R. T., Edwards, E., Dalal, S. S., Soltani, M.,
Nagarajan, S. S., Kirsch, H. E., Berger, M. S.,
Barbaro, N. M. and Knight, R. T., (2006). High
gamma power is phase-locked to theta oscillations in
human neocortex. Science, 313(5793), 1626–1628.
Cona, F., Zavaglia, M. and Ursino, M., (2012). Binding
and segmentation via a neural mass model trained with
hebbian and anti-hebbian mechanisms. International
Journal of Neural Systems, 22(02), 1-20.
Cowan, N., (2000). The magical number 4 in short-term
memory: A reconsideration of mental storage capacity.
Behavioral and Brain Sciences, 24(01), 87–185.
Doesburg, S. M., Green, J. J., McDonald, J. J. and Ward,
L. M., (2009). Rhythms of consciousness: binocular
rivalry reveals large-scale oscillatory network
dynamics mediating visual perception. PLoS ONE,
4(7), e6142.
Lisman, J., (2005). The theta/gamma discrete phase code
occuring during the hippocampal phase precession
may be a more general brain coding scheme.
Hippocampus, 15(7), 913–922.
von der Malsburg, C. and Buhmann, J., (1992). Sensory
segmentation with coupled neural oscillators.
Biological Cybernetics, 67(3), 233–242.
Miller, G. A., (1956). The magical number seven, plus or
minus two: some limits on our capacity for processing
information. Psychological Review, 63(2), 81–97.
Singer, W., (1999). Neuronal synchrony: a versatile code
for the definition of relations. Neuron, 24, 49–65.
Ursino, M., La Cara, G. E. and Sarti, A., (2003). Binding
and segmentation of multiple objects through neural
oscillators inhibited by contour information.
Biological Cybernetics, 89(1), 56–70.
Ursino, M., Cona, F. and Zavaglia, M., (2010). The
generation of rhythms within a cortical region:
Analysis of a neural mass model. NeuroImage, 52(3),
1080–1094.
Ursino, M., Magosso, E. and Cuppini, C., (2009).
Recognition of abstract objects via neural oscillators:
Interaction among topological organization,
associative memory and gamma band synchronization.
IEEE Transactions on Neural Networks, 20(2), 316–
335.
Varela, F., Lachaux, J. P., Rodriguez, E. and Martinerie,
J., (2001). The brainweb: Phase synchronization and
large-scale integration. Nature Reviews Neuroscience,
2(4), 229–239.
Wang, D., Buhmann, J. and von der Malsburg, C., (1990).
Pattern segmentation in associative memory. Neural
Computation, 2(1), 94–106.
Wang, D. and Terman, D., (1997). Image segmentation
based on oscillatory correlation. Neural Computation,
9(4), 805–836.
RecoveryofSequentialandNonSequentialMemorieswithaNeuralMassModel
551
