
Retinal Blood Vessel Segmentation by a MAS Approach
Carla Pereira
1
, Jason Mahdjoub
2
, Zahia Guessoum
2
, Luis Gonçalves
3
and Manuel Ferreira
1
1
Algoritmi Center, University of Minho, Campus de Azurém, 4800-058, Guimarães, Portugal
2
CReSTIC – MODECO, University of Reims, Rue des Crayères, 51100, Reims, France
3
Ophthalmology Service, Centro Hospitalar do Alto Ave, Guimarães, SA., 4835-044, Guimarães, Portugal
Keywords: Diabetic Retinopathy, Fundus Image Analyzing, Blood Vessels Segmentation, Multi-Agents System.
Abstract: Retinal blood vessels segmentation by color fundus images analysis has got huge importance for the
diabetic retinopathy early diagnosis. Several interesting computational approaches have been done in this
field, but none of them has shown the required performance due to the use of global approaches. Therefore,
a new approach is proposed based on an organization of agents enabling vessels detection. This multi-agent
approach is preceded by a preprocessing phase in which the fundamental filter is a Kirsch derivative
improved version. This first phase allows an environment construction where the agents are situated and
interact. Then, blood vessels segmentation emerges from agents’ interaction. According to this study,
competitive results were achieved comparing to those found in the present literature. It seems to be that a
very efficient system for the diabetic retinopathy diagnosis can be built using MAS mechanisms.
1 INTRODUCTION
Diabetic retinopathy (DR) has been presented as the
most common cause of blindness among working
age people. Retinal blood vessels segmentation has
got huge importance for the DR early diagnosis.
Numerous research efforts have been done in
segmenting blood vessels using image processing
techniques applied to fundus images. Some of the
approaches include matched filtering (Zhang et al.,
2010); machine-learning methods (Staal et al.,
2004), morphological operators (Mendonça and
Campilho, 2006). The main difficulties in vessels
accurate segmentation are pathologies presence,
noise, the low contrast between vasculature and
background, vessels width, brightness and shape
variability. To solve the variability problem, it is
important to adapt image interpretations, in loco,
instead of only applying one algorithm on the entire
image. A multi-agent system (MAS) is thus
proposed as a solution since agents allow several
algorithms cohabitation.
There are some studies reported in literature that
associate MAS to image processing in medical
images (Bovenkamp et al., 2004; Mahdjoub et al.,
2006; Richard et al., 2004). This association has
been revealed as a research expanded area. As far as
known, multi-agent approaches have never been
applied to retinal images. In this study, an approach
based on Mahdjoub et al. (2006) previous study is
applied to fundus images for the blood vessels
segmentation. This new approach uses some image
processing algorithms as concrete perception and
action tools to define autonomous agents which
interact among themselves and with environment
(image). Then blood vessel segmentation emerges as
a global behavior.
2 METHODOLOGY
The proposed approach uses a MAS model to
improve retinal blood vessels edges detection
resulting from a preprocessing phase. This
preprocessing phase consists of a conventional
image processing algorithms group providing
information (environment) for the MAS model.
2.1 Image Pre-processing
In this first step, we first use the preprocessing phase
from Niemeijer et al. (2005) developed study. In
order to remove noise from fundus image while
preserving the edges, Kuwahara filter (Kuwahara et
al., 1976) was then applied. Finally, a modified
Kirsch filter was employed to the image resulting
290
Pereira C., Mahdjoub J., Guessoum Z., Gonçalves L. and Ferreira M..
Retinal Blood Vessel Segmentation by a MAS Approach.
DOI: 10.5220/0004200602900293
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2013), pages 290-293
ISBN: 978-989-8565-36-5
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
from the last step. The Kirsch filtering is an image
edges enhancing method using a basic convolution
filter eight times rotated. The improved Kirsch filter
(Mahdjoub et al., 2006) enables edges detection with
a two pixels thickness whose external edge is
represented by a positive or negative value, whereas
the internal edge has an opposite value (Fig. 1 a).
This enables the MAS model detection process since
the blood vessels gradient reveals a specific pattern
(Fig. 1 b), as they can be represented by two parallel
linear segments series. Thus, the agents search for
blood vessels edges by looking for this gradient
specific pattern.
Figure 1: a) Modified Kirsch filter resultant image where
the blue and white pixels represent negative and positive
gradient values, respectively. b) One section image
expanded version of a).
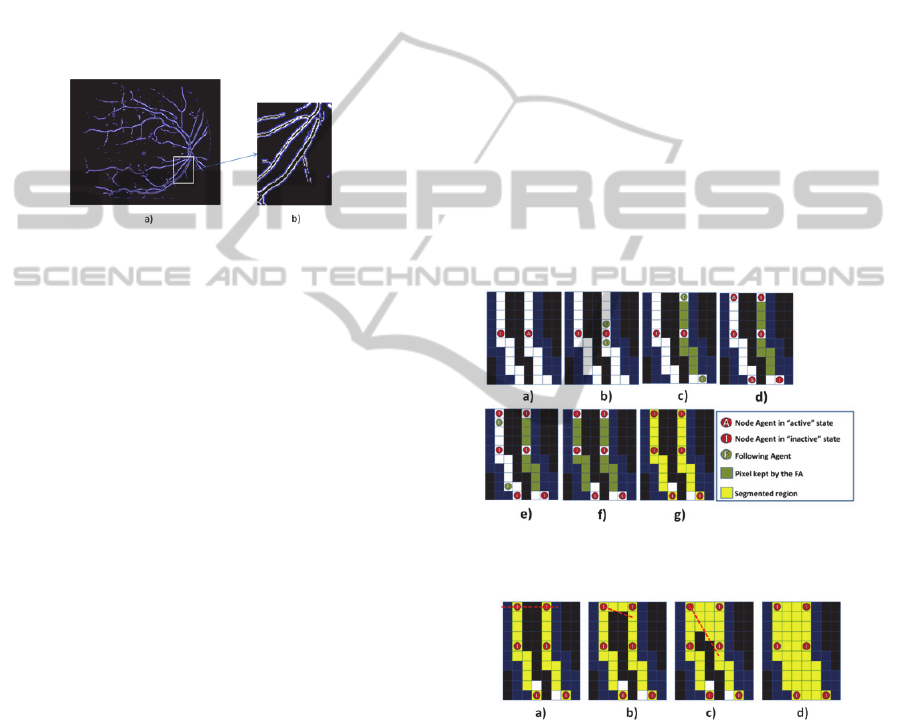
2.2 Multi-Agent System Model
MAS is composed by an agent set and their
environment. The environment contains the green
plane image in which each pixel contains the
intensity grey level and a Boolean value defining if
the pixel has already been explored by an agent.
Moreover, when located in the environment the
agents perceive the modified Kirsch gradient which
defines a right visible edge. Agents are of several
kinds with different behaviors according to their
current state and perception: search agents (SA),
following agents (FA), node agents (NA), and region
agents (RA).
MAS is initialized with a SA launched on one of
the white points from Fig. 1 a), randomly chosen.
This SA in the “operating” state has to find edges
belonging to blood vessel regions. It evolves in the
environment by following positive gradient points.
When it finds an edge, it initializes a new contour
and launches two NAs belonging to this contour:
one in the “active” state and another in the
“inactive” one (Fig. 2 a). Furthermore, the SA
changes its state to “suspended”. Then the “active”
NA has to allow contour extension and closure.
Therefore, it determines the possible directions to
follow the contour, creates FA and becomes
“inactive” (Fig. 2 b). FA follows the detected edge
until there is no direction to follow or until the
contour reaches a specific length (Fig. 2 c). FA
launches then an “inactive” NA on its position and
an “active” NA on the perpendicular direction where
it was moving (Fig. 2 d). Moreover, FA gives to the
“active” NA information about this direction
allowing it to launch another FA on the opposite
direction (Fig. 2 e). When this FA reaches the
initially launched “inactive” NA (Fig. 2 f) it
launches a RA (Fig. 2 g) which will be responsible
for the contour delimited region. RA sends a
message to SA to change its state to “operating” and
repeats all the process until all the blood vessels
contours are founded by MAS. There so, MAS
detects one contour each time avoiding regions
intersection at this phase. Afterwards SA sends a
message to all RAs to change their state to “filling”.
RA fills all the contours taking into account the
image grey levels (Fig. 3). Finally, RAs attempt
fusions with each other.
At the end of the process MAS has to reconstruct
the vessels by representing them with a succession
of regions initially represented by contours.
Figure 2: Contour formation graphical representation to
which a RA is assigned.
Figure 3: RA “filling” state graphical representation. It
analyses the pixels located between each pair of two
points belonging to its contour by determining the line
linking these two points a) – c); d) all the points that are
inside the contour, with grey level value similar to the
contour average grey level value, are added to the region.
3 RESULTS AND DISCUSSION
The proposed MAS model was implemented with
MadKit (Gutknecht and Ferber, 2000) and tested
RetinalBloodVesselSegmentationbyaMASApproach
291
with the publicly available DRIVE dataset (Staal et
al. 2004).
To measure the overall approach performance, it
is important to compare the resulting image with the
information detected by the Kirsch filter. Moreover,
the differences between the binary resultant image
with blood vessel segmentation and the ground truth
vessel map should be evaluated. In that way, three
common measurements, namely, sensitivity,
specificity and predictive value (Lalkhen and
McCluskey, 2008) were used for testing the
proposed algorithm. Fig. 4 and Fig. 5 show the
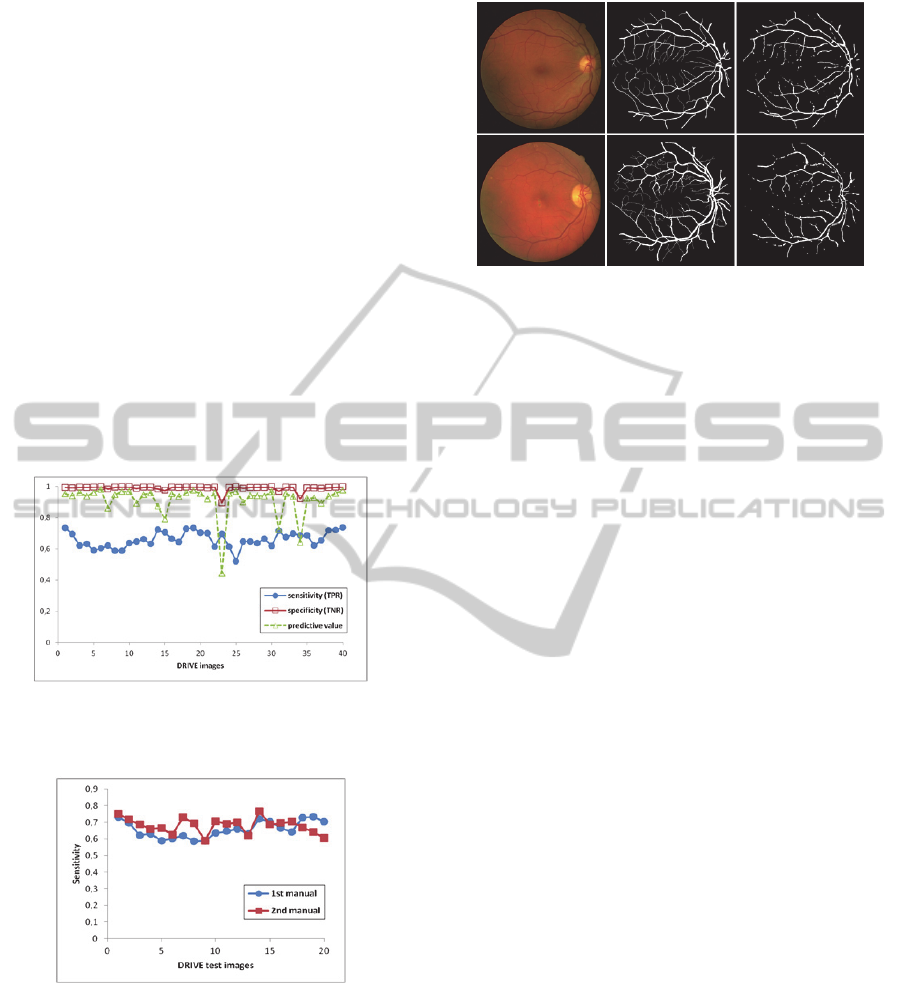
quantitative results obtained with this approach in
the DRIVE dataset. The proposed approach results
applied to normal retinal images are shown in Fig. 6.
These illustrate the original and resulting images
where the proposed approach had the best and the
worst performance respectively, with 73.6% and
51.9% sensitivity values and 97.4% and 99.7%
specificity values.
Figure 4: Sensitivity, specificity and predictive values
obtained for the 40 images of the DRIVE database with
the proposed approach.
Figure 5: Sensitivity values obtained for the 20 images of
the DRIVE database test set, using both hand labeled
databases.
Fig. 7 illustrates a superimposed image of the hand
labeled image with the hand labeled image after
morphological opening and with MAS result.
In this figure, the white pixels represent the pixels
common to the three images; the yellow and green
pixels represent the pixels that belong to blood
vessels but are not detected by MAS, that is, the
Figure 6: Images resulting where the proposed approach
had the best (above) and worst (below) performance in the
DRIVE database. From left to right: original color fundus
image; hand labeled image; blood vessel segmentation
using MAS approach.
false negative pixels; and the false positive pixels
are represented in blue. As it can be seen, the most
part of false positive pixels are located at the
manually segmented vessels border and therefore,
they should not be considered as false positive.
Actually, manual blood vessels segmentation from
retinal images is a very arduous and difficult task,
leading two people to segment the same image in
different ways. This can be observed in Fig. 5 where
two different hand labeled images for the same color
fundus images resulted in different sensitivity values
with the same approach.
Analyzing Fig. 6 (right) and Fig. 7 it can be
observed that MAS reconstructed the most part of
the vessels, especially the thickest ones. Some of the
thinnest vessels were also segmented but not all,
affecting the sensitivity values. In fact, after
removing the thinnest vessels from the hand labeled
image (green pixels of Fig. 7) the sensitivity values
of the proposed approach increased to values higher
than 80%. So, improvements have to be made in
MAS model to deal with the small vessels.
Moreover, there are some thickest vessels
portions not detected by MAS model mostly near the
FOV border and the optic disc contour. This last
problem may be related with the preprocessing
phase, mainly with Kuwahara filter since this often
produces clearly noticeable artifacts. (Papari, et al.,
2007).
Therefore, MAS is efficient in segmenting the
blood vessels from where the edges were already
detected in the preprocessing phase and in excluding
the detected pixels that did not belong to vessels.
Furthermore, MAS is able to close and aggregate
regions that were delimited by interrupted edges in
the kirsch resultant image, as a RA fusion process
result.
BIOSIGNALS2013-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
292

Figure 7: Hand labeled image superimposition with the
hand labeled image after the morphological opening and
with MAS result.
4 CONCLUSIONS
In this study, a MAS approach is proposed where
agents enrich a traditional edge detector algorithm.
The experiments show that the use of a MAS model
in the micro level could be an effective way to
segment structures in complex images such as retinal
images. In fact, through environment perception and
local interactions, a simple agent organization can
have as global behavior the most part of retinal
vasculature detection. The use of an improved
version of agent society with some knowledge a
priori about the retina proprieties, complemented
with some other traditional image processing
algorithms, could have the potential to develop a
system to detect and differentiate all the anatomic
and pathological structures of the fundus images.
Such an approach will overcome the classic image
processing algorithms that are limited to macro
results which cannot take into account the local
characteristics of a complex image. Therefore, it
could be a fundamental tool responsible for a very
efficient system development to be used in screening
programs concerning DR diagnosis.
ACKNOWLEDGEMENTS
Work supported by FEDER funds through the
"Programa Operacional Factores de Competitividade
– COMPETE" and by national funds by FCT-
Fundação para a Ciência e a Tecnologia. C. Pereira
thanks the FCT for the SFRH/BD/61829/2009 grant.
REFERENCES
Bovenkamp, E.G.P., Dijkstra, J., Bosch, J.G., and Reiber,
J. H. C. (2004). Multi-agent segmentation of IVUS
images. Pattern Recognition, 37:647-663.
Gutknecht, O., Ferber, J., (2000). Madkit: a generic multi-
agent platform. In: Proceedings of the 4th
international conference on Autonomous agents,
Barcelona, Spain, 78-79.
Kuwahara, M. , Hachimura, K., Eiho, S., and Kinoshita,
M. (1976) Digital Processing of Biomedical Images
(pp. 187-203). Plenum Press: New York.
Lalkhen, A. G., McCluskey, A. (2008). Statistics VI:
Clinical tests: sensitivity and specificity. Contin Educ
Anaesth Crit Care Pain, 8(6):221-223.
Mahdjoub, J., Guessoum, Z., Michel, F., and Herbin, M.
(2006). A multi-agent approach for the edge detection
in image processings. in 4th European Workshop on
Multi-Agent Systems, Lisbon, Portugal.
Mendonça, A., and Campilho, A. (2006). Segmentation of
retinal blood vessel by combining the detection of
centerlines and morphological reconstruction. IEEE
Trans. Med. Imag., 25(9):1200-1213.
Niemeijer, M., Ginneken, B. van, Staal, J., Suttorp-
Schulten, M., and Abràmoff, M.D.(2005). Automatic
detection of red lesions in digital color fundus
photographs. IEEE Trans. Med. Imag., 24(5): 584-
592.
Papari, G., Petkov, N., Campisi, P., (2007). Artistic edge
and corner enhancing smoothing. IEEE Transactions
on Medical Imaging 16 (10):2449 - 2462.
Richard, N., Dojat, M., and Garbay, C. (2004). Distributed
Markovian segmentation: Application to MR brain
scans. Pattern Recognition, 40:3467 – 3480.
Staal, J. J., Abrámoff, M. D., Niemeijer, M., Viergever, M.
A., and Ginneken, B. van (2004). Ridge-based vessel
segmentation in color images of the retina. IEEE
Trans. Med. Imag., 23(4): 501-509.
Zhang, B., Zhang, L., Zhang, L., and Karray, F. (2010).
Retinal vessel extraction by matched filter with first-
order derivative of Gaussian. Computers in Biology
and Medicine, 40:438-445.
RetinalBloodVesselSegmentationbyaMASApproach
293
