
Portable Custom Built Device for Thermal Sensitivity Assessment
An Auxiliary Tool to Characterize the Neuropathic Pain following Spinal Cord
Injury
Renato Varoto
1
, Fábio Casagrande Hirono
1
, Fernando Ometto Zorzenoni
2
,
Ricardo Yoshio Zanetti Kido
2
, William Barcellos
1
and Alberto Cliquet Jr.
1,2
1
Department of Electrical Engineering, University of São Paulo (USP), São Carlos, Brazil
2
Department of Orthopedics and Traumatology, University of Campinas (UNICAMP), Campinas, Brazil
Keywords: Thermal Sensitivity Assessment, Neuropathic Pain, Spinal Cord Injury.
Abstract: Neuropathic pain is characterized to arise without stimulation of nociceptors, but due to injury or
dysfunction of Peripheral and Central Nervous Systems. It involves altered mechanisms of impulse
transmission in somatosensory pathways, causing abnormal sensations. Quantitative sensory testing, by the
detection of thermal stimuli, is a method used to characterize and study the neuropathic pain. Therefore, this
work describes the development and application of portable custom built device for cutaneous thermal
sensitivity assessment in spinal cord injured subjects (SCIS). Using method of levels, the assessment was
applied in healthy subjects and SCIS with and without neuropathic pain. The thresholds determined for
healthy subjects during thermal sensitivity assessment are consistent and other results provided by clinical
trials are according to previous works, demonstrating the device feasibility as an auxiliary tool for
neuropathic pain study.
1 INTRODUCTION
Spinal cord injury (SCI) causes disruption of nerve
fibres that transmit ascending sensory and
descending motor information. This disruption
causes losses in the transmission of sensory-motor
information across the site of the lesion, resulting in
considerable physical and emotional consequences
for individual (Maynard Jr. et al., 1997); (Eng and
Miller, 2006). Sensory-motor dysfunctions occur in
the parts of the body innervated by areas below the
site of the lesion, being characterized by paralysis,
altered sensation and weakness (Raineteau and
Schwab, 2001).
Spinal cord injured subjects (SCIS) also suffer
other disorders and numerous secondary pathologies
such as losses of bowel and bladder functions,
pressure ulcers, spasticity, gastrointestinal and
sexual dysfunctions and heterotopic ossification
(Kaplan et al., 1991); (Eng and Miller, 2006);
(Verschueren et al., 2011). However, one of the
major problems following SCI is the neuropathic
pain (Bonica, 1991).
Neuropathic pain is characterized to arise
without stimulation of nociceptors (sensory pain
fibres that detect tissue damage by physical,
chemical or thermal phenomena), but due to injury
or dysfunction of Peripheral and Central Nervous
Systems. Thus, neuropathic pain is an aggravating
for the already weakened patient, imposing severe
limitations in performing the activities of daily
living (Richards et al., 1980); (Summers et al.,
1991).
The pathophysiology of neuropathic pain
involves altered mechanisms of impulse
transmission in somatosensory pathways, so that
axonal injury leads to a gain in excitatory
transmission, in other words, there is a massive
axonal input. It results from an axonal
hyperexcitability, with the generation of ectopic
electrical impulses, causing abnormal sensations
(Catafau and Bosque, 2003).
In SCIS, partially preserved pathways
spinothalamic tract may be the local generator of
pain (Wasner et al., 2008). Fibres Aδ and C present
little myelin and follow the column via anterolateral
spinothalamic tract. These fibres are the main
components of the fibres that lead thermal sensitivity
(Kirillova et al., 2011) Thus, the thermal sensitivity
28
Varoto R., Casagrande Hirono F., Ometto Zorzenoni F., Yoshio Zanetti Kido R., Barcellos W. and Cliquet Jr. A..
Portable Custom Built Device for Thermal Sensitivity Assessment - An Auxiliary Tool to Characterize the Neuropathic Pain following Spinal Cord Injury.
DOI: 10.5220/0004225900280034
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 28-34
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
follows the same neurological path of the pain.
Some methods are applied to characterize and
study the neuropathic pain, for example, McGill
Pain Questionnaire, quantitative sensory testing
(QST) and somatosensory evoked potential
(Finnerup et al., 2003).
The McGill Pain Questionnaire is an instrument
that evaluates qualitatively and quantitatively pain,
providing quantitative measures of clinical pain that
can be treated statistically (Melzack, 1975). Pimenta
and Teixeira (1996) adapted (translation and
validation) the questionnaire to Portuguese. The
present pain intensity (PPI) is the number chosen by
the subject at the time of administration of the
questionnaire, ranging from 0 (no pain) to 5
(excruciating). The pain rating index based on the
subjects’ mean scale values (PRI(S)) obtained by
Melzack and Torgerson (1971) is described as the
sum of all values of words chosen by subject for all
categories (sensory, affective, evaluative, motor and
miscellaneous). And other important value is the
number of words chosen (NWC) that is the sum of
all words chosen by the subject (Melzack, 1975).
QST assess and quantify sensory function in
subjects with losses in the neurological system,
measuring the detection threshold of tactile,
vibratory, thermal or painful stimuli (Shy et al.,
2003); (Finnerup et al., 2003). Especially for thermal
stimuli, some equipments utilize the Peltier effect, in
which the intensity and direction of electrical current
controls the surface temperature of a test electrode.
The skin was touched by the electrode and the
subject reports the sensation in relation to the
temperature (Shy et al., 2003); (Kenshalo and
Bergen, 1975); (Finnerup et al. 2003).
This paper describes the development and
application of portable custom built device for
cutaneous thermal sensitivity assessment based on
Peltier effect. This device is designed for quick and
practice assessment of thermal sensitivity in SCIS,
representing an auxiliary tool for neuropathic pain
study. Using method of levels, the device was used
in healthy subjects and SCIS with and without
neuropathic pain. In general, the obtained results
were compared with previous works to verify the
device feasibility.
2 MATERIALS AND METHODS
About this work, instrumentation development was
done at Laboratory of Biocybernetics and
Rehabilitation Engineering - USP, and clinical
application was performed at Laboratory of
Biomechanics and Rehabilitation of the Locomotor
System – UNICAMP.
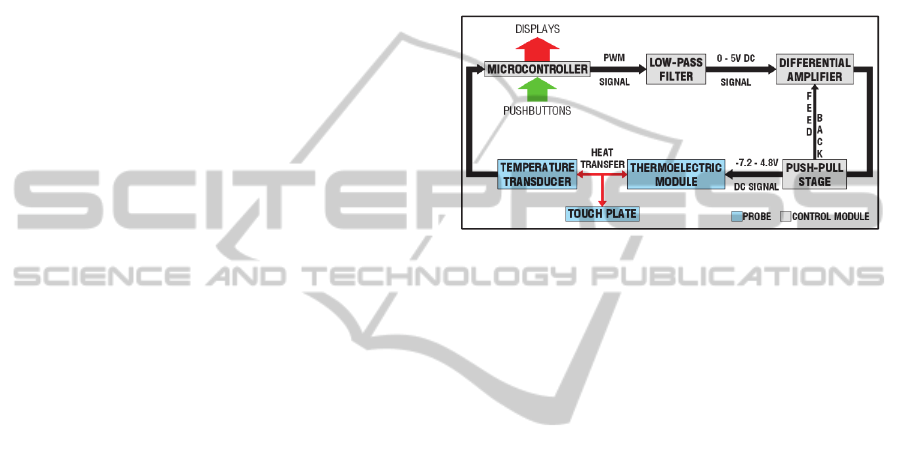
Basically, portable custom built device is
composed by microcontroller, thermoelectric
module and temperature transducer. The
microcontroller associated with amplifier circuits
offers electrical energy to supply the thermoelectric
module and provides information about device
operation condition. Furthermore, it allows setting
the probe operating temperature (Figure 1).
Figure 1: Block diagram of the device.
2.1 Thermoelectric Module,
Temperature Transducer and
Probe Assembly
The thermoelectric module used was a solid state
heat pump (Melcor Corporation, Trenton, NJ, USA),
based on Peltier effect. This heat pump contains 66
thermocouples, being able to transfer until 3.56W of
heat from cold to hot faces (Q
max
); it results in the
maximum temperature difference of the 67
o
C
between two faces (∆T
max
) with low power
consumption - input electrical current (I
max
) of 0.8A
and dc voltage (V
max
) of 7.98V – to achieve ∆T
max
.
Temperature transducer with analog output based
on semiconductor junctions was used to monitor the
temperature of thermoelectric module. The
transducer used was LM35 (National Semiconductor
Corporation, Santa Clara, CA, USA) that is a
precision integrated-circuit temperature transducer,
whose output voltage is linearly proportional to the
Celsius temperature. The LM35 does not require
external calibration to provide readouts with
accuracies of ±0.75
o
C over a range -55 to +150
o
C.
Other important features of LM35 make it suitable
for control circuits as low output impedance, very
low self-heating and sensitivity of 0.01V/
o
C.
The probe is composed by aluminium touch plate
(16x16mm), thermoelectric module, LM35
transducer, heat sink and auxiliary fan. In the first
stage of probe assembly, LM35 was coupled to the
touch plate by aluminium clamp, and the
PortableCustomBuiltDeviceforThermalSensitivityAssessment-AnAuxiliaryTooltoCharacterizetheNeuropathic
PainfollowingSpinalCordInjury
29
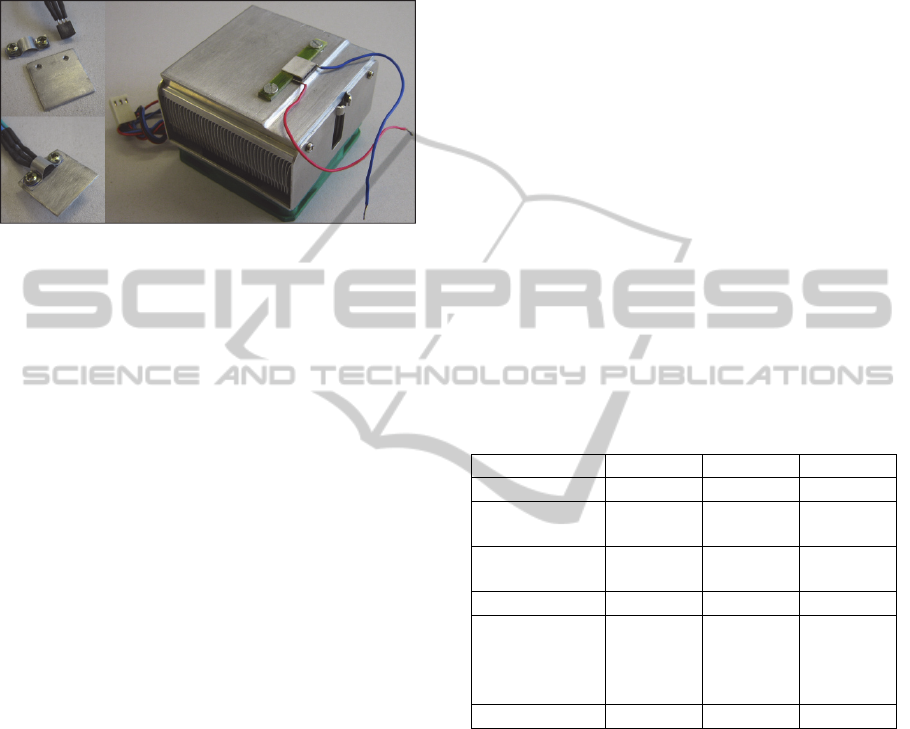
thermoelectric module was fixed on heat sink
(Figure 2). Touch plate with LM35 was fixed on
thermoelectric module by thermal compound, in the
second stage. Moreover, the assembly was placed in
a suitable case.
Figure 2: First stage of probe assembly.
2.2 Electronic Apparatus
The microcontroller used was PIC18F252
(Microchip Technology Inc., Chandler, AZ, USA)
and it was programmed to use pulse-width
modulation (PWM) as technique for controlling
power to thermoelectric module. It compares the
desired temperature of the probe with the actual
temperature and, in accordance with this difference,
adjusts the duty cycle of PWM signal.
An active low-pass filter was used to generate a
dc signal from 0 – 5V PWM signal. This filter was
configured in Sallen-Key topology of second order
with cut-off frequency of approximately 35 Hz and
quality factor of 0.5.
During bench tests with the probe, dc voltages of
thermoelectric module to ensure that the temperature
probe reached 0 and 60
o
C were determined. The
voltage polarity was defined so that a negative
voltage decreases the probe temperature and a
positive one would increase the temperature. These
voltages were -7.2V and 4.8V, respectively.
Therefore, it was necessary to convert 0 – 5V dc
signal (Vin) obtained from PWM into asymmetric
bipolar dc signal (-7.2V – 4.8V) (Vout) required by
thermoelectric module. This conversion was done by
differential amplifier using an operational amplifier
as active component, whose inverting input was held
at 5V. Thus, an amplifier with linear transfer
characteristic was developed (1), resulting in proper
range of signal.
Vout=2.435*Vin - 7.353 (1)
Although the range of signal was appropriate, the
differential amplifier was not able to provide the
required electrical power (up to 5.76W). Thus, a
push-pull stage with MOSFETs was applied. These
components exhibit a negative thermal coefficient,
in other words, its electrical conductivity decreases
with increasing temperature, protecting the
electronic circuit.
The output of push-pull stage with unitary gain
was also used as feedback signal to differential
amplifier. This strategy reduces signal distortion,
providing a linear signal to thermoelectric module.
Whole electronic circuit including the thermoelectric
module is powered by two batteries (12V, 5Ah).
2.3 Clinical Trials
Twenty SCIS were recruited to participate in this
work, and they were divided into two groups, pain
SCIS (P) and non-pain SCIS (NP). Subjects were
classified according to the American Spinal Cord
Injury Association (ASIA) Impairment Scale (AIS)
(Maynard Jr. et al., 1997); (Dahlberg et al., 2005);
(Wolfe and Hsieh, 2006); (Kirshblum et al., 2011).
Control group (CT) was formed by 10 healthy
subjects (Table 1).
Table 1: Subjects characteristics.
P NP CT
Age (year)*
39.3(12.1) 35.4(12.9) 27.2(10.6)
Gender
(Male/Female)
9/1 7/3 9/1
Body mass
(kg)*
69.2(10.1) 71.5(14.7) 77.6(13.6)
Height (m)*
1.74(0.07) 1.72(0.09) 177.3(4.8)
Neurological
lesion level
(Cervical/
Thoracic)
7/3 3/7 -
AIS (A/B/C/D)
8/2/0/0 7/2/1/0 -
*Values in mean(SD)
Inclusion criteria for P group were lesion level
above T12 with central neuropathic pain after
traumatic SCI. Exclusion criteria were based on the
presence of any other pain different from central
neuropathic pain such as nociceptive or peripheral
neuropathic pain; or subjects that were under
analgesic treatment.
In relation to NP group, inclusion criteria were
lesion level above T12 without central neuropathic
pain and spontaneous dysesthesia.
To study the sensitivity of pain and losses of
sensory pathways following SCI, McGill Pain
Questionnaire (Portuguese version) and thermal
sensitivity assessment were applied.
For McGill Pain Questionnaire, the values used
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
30
for data analysis were PRI, NWC and PPI.
The thermal stimuli were applied to the dominant
leg at a point 100mm distal from the patella, in the
anterolateral side of the leg, corresponding to the L5
dermatome. For temperature range from 30
o
C to
60
o
C, with increment of 5
o
C, the skin was stimulated
by probe (aluminium touch plate, specifically) by
over 3s. Subsequently, the temperature range was
from 30
o
C to 0
o
C, with decrement of 5
o
C. Warm and
cold thresholds (temperature at which the patient
feels the stimulus) and pain thresholds were
recorded using the method of levels (Shy et al.,
2003). For each subject, three measurements with
interstimulus interval of 3 – 6s were used to
calculate the thresholds.
3 RESULTS
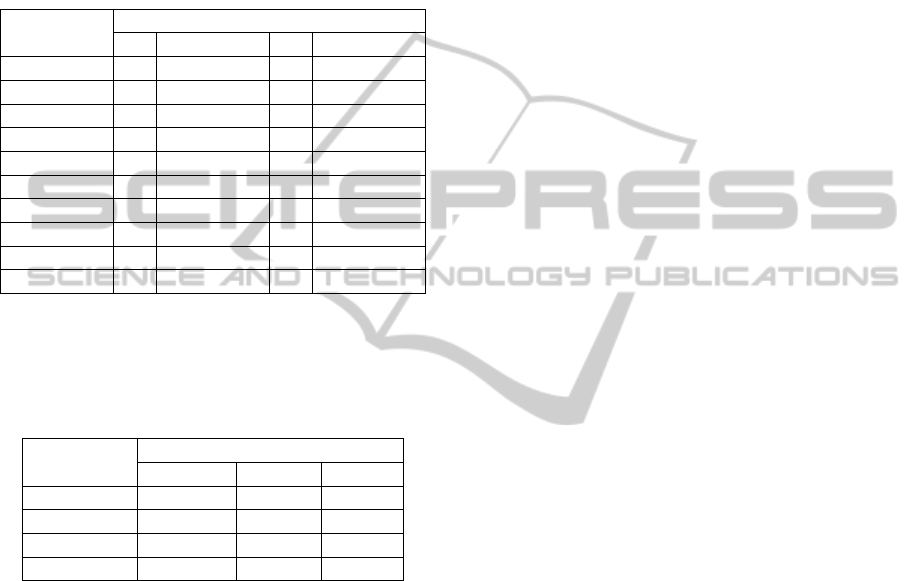
Figure 3 shows portable custom built device for
thermal sensitivity assessment; probe and control
module.
Figure 3: Portable custom built device for thermal
sensitivity assessment.
On the front panel, the control module has two
pushbuttons that set the desired probe temperature;
the red pushbutton (+) increases probe temperature
of 1
o
C while the black one (-) decreases it of 1
o
C, at
range of 0
o
C to 70
o
C. This desired probe
temperature and the instantaneous one are shown on
the smaller green display and larger red display,
respectively. When temperatures become equal,
LED turns on, indicating that probe is ready to use.
Besides, the front panel has a toggle switch for the
auxiliary fan and a DB9 connector for the probe
cable.
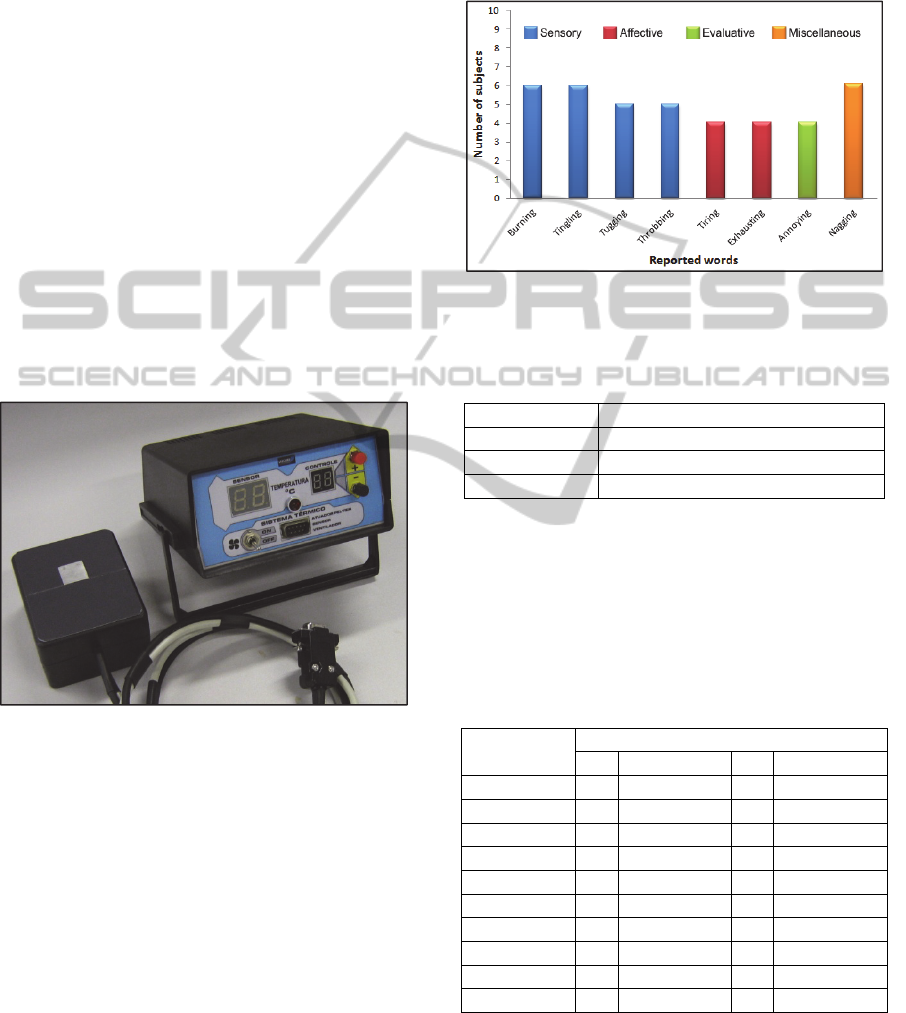
According to the McGill Pain Questionnaire
applied to the P group, half of subjects feel pain at
injury level and half of them below the injury level.
Figure 4 shows the relation between reported words
and number of subjects for each group of
questionnaire; and table 2 presents the scores for
each variable.
Figure 4: Relation between reported words and number of
subjects for each group of McGill Pain Questionnaire
(Portuguese version).
Table 2: McGill pain questionnaire scores.
Variables McGill Pain Questionnaire scores
PRI
28.5(13.7)
NWC
12.7(3.0)
PPI
3.2(1.4)
*Values in mean(SD)
During thermal sensitivity assessment, three
subjects detected cold, two detected warm and one
detected pain due to heat, in the NP group (Table 3).
Furthermore, none reported pain due to cold and one
subject (AIS C) presented muscle spasms with
stimulus of 50
o
C.
Table 3: Thermal sensitivity in the NP group.
Subjects (AIS)
Detected threshold ▲
Cold Pain due to cold Warm Pain due to heat
1(A) - - - -
2(B) - - - -
3(B) ▲ - ▲ ▲
4(A) - - - -
5(A) ▲ - - -
6(A) - - - -
7(A) - - - -
8(A) - - - -
9(A) ▲ - ▲ -
10(A) - - - -
In the P group, four subjects detected cold, one
detected pain by cold at 5°C and seven subjects
detected warm. Four subjects detected pain due to
heat and heat pain tolerance, with three subjects (all
PortableCustomBuiltDeviceforThermalSensitivityAssessment-AnAuxiliaryTooltoCharacterizetheNeuropathic
PainfollowingSpinalCordInjury
31
AIS A) presenting muscular spasms at 55°C (Table
4). One subject (AIS B) felt dysesthesia at the
stimulation site with stimuli of 0ºC and 45°C.
Another subject (AIS A) detected warm all around
the knee (not only at the stimulation site) detecting
warm at 0°C. And one subject (AIS A) felt a non
specific vibration in the L5 dermatome with 45°C in
the right leg.
Table 4: Thermal sensitivity in the P group.
Subjects (AIS)
Detected threshold ▲
Cold Pain due to cold Warm Pain due to heat
1(A) - - ▲ ▲
2(B) - - ▲ -
3(B) ▲ - ▲ ▲
4(A) - - - -
5(A) - - ▲ -
6(A) ▲ - ▲ ▲
7(A) ▲ - ▲ -
8(A) - - - -
9(A) - - - -
10(A) ▲ ▲ ▲ ▲
Table 5 indicates cold (C), warm (W), pain due
to heat (HP) and heat pain tolerance (HPT)
thresholds for each group.
Table 5: Temperature thresholds for each group.
Threshold(
o
C)
Group
P NP CT
C
15(9.8) 20.6(9.5) 19.2(5.2)
W
38.3(11.9) 38.3(2.6) 36.4(3.3)
HP
47.9(5.4) 50(0) 50.8(2.6)
HPT
48.3(4.9) 50(0) 52.8(3.1)
Values in mean(SD)
4 DISCUSSION
For touch plate construction, aluminium, copper and
stainless steel were available. The choice was based
on coefficient of thermal conductivity and oxidation
resistance of metals. According to the coefficient of
thermal conductivity, copper (398W/mK) is a better
conductor than aluminium (247W/mK) and stainless
steel (15.9W/mK), but stainless steel presents high
oxidation resistance. Therefore, aluminium was
chosen because presents high coefficient of thermal
conductivity and intermediate oxidation resistance
(Callister Jr., 2001).
These properties associated to the low mass and
small dimension of touch plate (0.7g) enabled a fast
thermal equilibrium between both plate surfaces.
Thus, the surface temperature acquired by the
transducer is the same of surface dedicated to the
touch.
Reach and stabilization of desired probe
temperature can be attributed to the technique for
controlling power to thermoelectric module and the
use of heat sink and auxiliary fan. For each
increment or decrement of 5
o
C, this strategy allowed
temperature stabilization in around 5s during clinical
trials.
In relation to the technique for controlling power
to thermoelectric module, another alternative based
on the use of PWM signal and an H-bridge can be
applied, replacing low-pass filter and differential
amplifier. However, this configuration provides a dc
voltage range from -12V to 12V for the PWM duty
cycle of 0 and 100%, respectively; values which are
not in accordance with the asymmetric bipolar dc
signal (-7.2V – 4.8V) required by the thermoelectric
module to operate in proper temperature range (0 –
60
o
C). Thus, the PWM duty cycle should be limited
between values of 20% and 70%, also avoiding
damage to the module since this operates at a
maximum dc voltage of 7.98V. Therefore, the use of
the low-pass filter and the differential amplifier is
more appropriate to the objectives of this work.
Generally, in healthy subjects, the activity of
cold-sensitive neurons increases below 35
o
C, and
maximum cutaneous cold sensitivity is around 25
o
C,
while cold fibre activity is ceased at temperatures
below 12
o
C. The firing rates of warm-sensitive
neurons increase above 25
o
C, and their range of
thermosensitivity extends from 35
o
C to 43
o
C.
Temperatures above 43
o
C and below 12
o
C cause
pain, whose stimulus is transmitted by Aδ and C
fibres. Furthermore, nociceptive heat activates Aδ
fibres around 43
o
C, while temperatures above 52
o
C
activate C fibers. Cold stimuli below 12
o
C also
cause pain and, in addition, nociceptives heat and
cold are transmitted by polymodal C fibers (Nomoto
et al., 2004; Schepers and Ringkamp, 2010).
Therefore, the thresholds determined for CT group
during thermal sensitivity assessment using portable
custom built device are consistent (Table 3).
In relation to SCIS, P group was more sensitive
to thermal stimuli than NP group, where 70% of
subjects in P group detected some kind of thermal
sensitivity against 30% of NP group (Tables 3 and
4). This difference between P and NP groups is in
agreement with the study of Wasner et al. (2008). In
this study, it was reported that there is some
preservation of the spinothalamic tract in pain SCIS
greater than in non pain SCIS, which may be
involved in the development of neuropathic pain.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
32

From the three subjects who experienced thermal
stimuli in NP group, only one presents complete
injury. For the P group, two subjects are AIS B and
five are AIS A.
This finding can be justified through theories that
explain why subjects with complete SCI have some
sensibility, characterizing the discomplete injury.
Dimitrijevic (1988) and Sherwood, Dimitrijevic and
Mckay (1992) found motor remnants in complete
SCIS due to a neural control. Thus, discomplete
injury is an incomplete injury that fits the AIS
criteria for grade A. Moreover, some subjects with
complete SCI can present some semblance of
sensibility, which can be evoked below the level of
injury due to incomplete injuries in the
spinothalamic tract. These subjects have subclinical
functions of ascending and descending tracts
(Finnerup et al., 2004).
5 CONCLUSIONS
Due to feedback control, the custom built portable
device provides easy temperature control with
resolution of 1
o
C. The device is simple to build and
can stabilize its temperature in about 5s for a 5
o
C
temperature change, therefore representing a simple
alternative for quick and practical assessment. It can
provide quantified information about sensory
performance of subjects, and the results obtained
from clinical trials are in accordance to previous
works, thus demonstrating the device feasibility for
thermal sensitivity assessment. Spinal cord injured
subjects that refer neuropathic pain are more
sensitive to thermal stimuli than patients do not
present neuropathic pain.
ACKNOWLEDGEMENTS
We thank the support by grants from São Paulo
Research Foundation (FAPESP) and National
Council for Scientific and Technological
Development (CNPq).
REFERENCES
Bonica, J. J., 1991. Introduction: semantic, epidemiologic,
and educational issues. In: Casey, K. L. Pain and
Central Nervous System Disease: the Central Pain
Syndromes. Raven Pr. New York: Raven Pr, pp 13–
29.
Callister Jr., W. D., 2001. Fundamentals of Material
Science and Engineering. 5
th
ed. New York: John
Wiley & Sons, Inc.
Catafau, S., Bosque, Q., 2003. Mecanismos
fisiopatológicos da dor neuropática, 1
st
ed. Madrid:
Medica Panamericana.
Dahlberg, A., Alaranta, H., Sintonen, H., 2005. Health-
related quality of life in persons with traumatic spinal
cord lesion in Helsinki. Journal of Rehabilitation
Medicine, 37, pp. 312–316.
Dimitrijevic, M. R., 1988. Residual motor functions in
spinal cord injury. Advances in Neurology, 47, pp.
138–155.
Eng, J. J., Miller, W. C., 2006. Rehabilitation: from
bedside to community following spinal cord injury
(SCI). In: Eng, J. J., Teasell, R. W., Miller, W. C.,
Wolfe, D. L., Townson, A. F., Aubut, J., Abramson,
C., Hsieh, J. T. C., Connolly, S. Spinal Cord Injury
Rehabilitation Evidence. Vancouver, pp. 16–29.
Finnerup, N. B., Johannesen, I. L., Fuglsang-Frederiksen,
A., Bach, F. W., Jensen, T. S., 2003. Sensory function
in spinal cord injury patients with and without central
pain. Brain, 126, pp. 57–70.
Finnerup, N. B., Gyldensted, C., Fuglsang-Frederiksen,
A., Bach, F. W., Jensen, T. S., 2004. Sensory
perception in complete spinal cord injury. Acta
Neurologica Scandinavica, 109, pp. 194–199.
Kaplan, S. A., Chancellor, M. B., Blaivas, J. G., 1991.
Bladder and sphincter behavior in patients with spinal
cord lesions. Journal of Urology, 146, pp. 113–117.
Kenshalo, D. R., Bergen, D. C., 1975. A device to
measure cutaneous temperature sensitivity in humans
and subhuman species. Journal of Applied Physiology,
39, pp. 1038–1040.
Kirillova, I., Rausch, V. H., Baron, R., Jänig, W., 2011.
Mechano- and thermosensitivity of injured muscle
afferents. Journal of Neurophysiology, 105, pp. 2058–
2073.
Kirshblum, S. C., Burns, S. P., Biering-Sorensen, F.,
Donovan, W., Graves, D. E., Jha, A., Johansen, M.,
Jones, L., Krassioukov, A., Mulcahey, M. J., Schmidt-
Read, M., Waring, W. 2011. International standards
for neurological classification of spinal cord injury
(Revised 2011). The Journal of Spinal Cord Medicine,
34, pp. 535–546.
Maynard Jr., F. M., Bracken, M. B., Creasey, G., Ditunno
Jr, J. F., Donovan, W. H., Ducker, T. B., Garber, S. L.,
Marino, R. J., Stover, S. L., Tator, C. H., Waters, R.
L., Wilberger, J. E., Young, W., 1997. International
standards for neurological and functional classification
of spinal cord injury. Spinal Cord, 35, pp. 266–274.
Melzack, R., 1975. The McGill Pain Questionnaire: major
properties and scoring methods. Pain, 1, pp. 277–299.
Melzack, R., Torgerson W. S., 1971. On the language of
pain. Anesthesiology, 422, pp. 50–59.
Nomoto, S., Shibata, M., Iriki, M., Riedel, W., 2004. Role
of afferent pathways of heat and cold in body
temperature regulation. International Journal of
Biometeorology, 49, pp. 67–85.
Pimenta, C. A. de M., Teixeira, M. J., 1996. Questionário
PortableCustomBuiltDeviceforThermalSensitivityAssessment-AnAuxiliaryTooltoCharacterizetheNeuropathic
PainfollowingSpinalCordInjury
33

de dor McGill: proposta de adaptação para a língua
portuguesa. Revista da Escola de Enfermagem da
USP, 30, pp. 473–483.
Raineteau, O., Schwab, M. E., 2001. Plasticity of motor
systems after incomplete spinal cord injury. Nature
reviews. Neuroscience, 2, pp. 263–273.
Richards, J. S., Meredith, R. L., Nepomuceno, C., Fine, P.
R., Bennett, G., 1980. Psychosocial aspects of chronic
pain in spinal cord injury. Pain, 8, pp. 355–408.
Schepers, R. J., Ringkamp, M., 2010. Thermoreceptors
and thermosensitive afferents. Neuroscience and
Biobehavioral Reviews, 34, pp. 177–184.
Sherwood, A. M., Dimitrijevic, M. R., Mckay, W. B.,
1992. Evidence of subclinical brain influence in
clinically complete spinal cord injury: discomplete
SCI. Journal of the Neurological Sciences, 110, pp.
90–98.
Shy, M. E, Frohman, E. M., So, Y. T., Arezzo, J. C.,
Cornblath, D. R., Giuliani, M. J., Kincaid, J. C.,
Ochoa, J. L., Parry, G. J., Weimer, L. H., 2003.
Quantitative sensory testing. Neurology, 60, pp. 898–
904.
Summers, J. D., Rapoff, M. A., Varghese, G., Porter, K.,
Palmer, R. E., 1991. Psychosocial factors in chronic
spinal cord injury pain. Pain, 47, pp. 183–189.
Verschueren, J. H. M., Post, M. W. M., de Groot, S., van
der Woude, L. H. V., van Asbeck, F. W. A., Rol, M.,
2011. Occurrence and predictors of pressure ulcers
during primary in-patient spinal cord injury
rehabilitation. Spinal Cord, 49, pp. 106–112.
Wasner, G., Lee, B. B., Engel, S., Mclachlan, E., 2008.
Residual spinothalamic tract pathways predict
development of central pain after spinal cord injury.
Brain, 131, pp. 2387–2400.
Wolfe, D. L., Hsieh, J. T. C., 2006. Rehabilitation practice
and associated outcomes following spinal cord injury.
In: Eng, J. J., Teasell, R. W., Miller, W. C., Wolfe, D.
L., Townson, A. F., Aubut, J., Abramson, C., Hsieh, J.
T. C., Connolly, S. Spinal Cord Injury Rehabilitation
Evidence. Vancouver, pp. 44–90.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
34
