
Translation Efficiency of Synaptic Proteins and Its Coding Sequence
Determinants
Shelly Mahlab
1
, Itai Linial
2
and Michal Linial
3
1
School of Computer Science and Engineering, The Hebrew University of Jerusalem, 91904, Israel
2
The Racah Institute of Physics, The Hebrew University of Jerusalem, 91904, Israel
3
Department of Biological Chemistry, Institute of Life Sciences, The Hebrew University of Jerusalem, 91904, Israel
Keywords: Translation Rate, Codon Usage, Neuron, tRNA Adaptation, Endocytosis, Local Translation, Dendrites.
Abstract: The synapse is an organized structure that contains synaptic vesicles, mitochondria, receptors, transporters
and stored proteins. About 10% of the mRNAs that are express in mammalian neurons are delivered to
synaptic sites, where they are subjected to local translation. While neuronal plasticity, learning and memory
occur at the synapse, the mechanisms that regulate post-transcriptional events and local translation are
mostly unknown. We hypothesized that evolutional signals that govern translational efficiency are encoded
in the mRNA of synaptic proteins. Specifically, we applied a measure of tRNA adaptation index (tAI) as an
indirect proxy for translation rate and showed that ionic channels and ligand-binding receptors are specified
by a global low tAI values. In contrast, the genuine proteins of the synaptic vesicles exhibit significantly
higher tAI values. The expression of many of these proteins actually accompanied synaptic plasticity.
Furthermore, in human, the local tAI values for the initial segment of mRNA coding differs for synaptic
proteins in view of the rest of the human proteome. We propose that the translation of synaptic proteins is a
robust solution for compiling with the high metabolic demands of the synapse.
1 INTRODUCTION
Translation must be tightly controlled for coping
with the cell demand and its limited resources.
Energetically, translation is the most expensive
operation in dividing cells (Arava, et al., 2003;
Gingold and Pilpel, 2011; Ingolia, et al., 2009).
Thus, an appropriate regulation of the rate of
translation reduces the ribosomal drop-off, the
translation errors and improves the overall ribosomal
allocation (Zhang, et al., 2010).
In unicellular organisms, it has been shown that
the genomic tRNA copy number (CN) approximates
the levels of intracellular tRNA and thus the codon
usage. Moreover, the relative genomic abundance of
synonymous codons varies in all organisms from
bacteria to mammals (Sharp, et al., 1993), and codon
usage among genes tends to be related to their
expression levels (dos Reis, et al., 2004; Marais and
Duret, 2001; Plotkin and Kudla, 2010; Tuller, et al.,
2010). Specifically, highly expressed genes (e.g,
ribosomal proteins) tend to include codons that are
recognized by abundant tRNA molecules,
suggesting that the control of the translation process
is under a selective pressure.
In all organisms, decoding of mRNA to proteins
occurs by tRNAs. The tRNA anticodon recognizes
the complementary codon or the wobble-based
codon that encodes the same amino acid (Percudani,
2001). In bacteria and fungi, the genomic tRNA CN
correlates with the intracellular tRNA levels
(Ikemura, 1985; Lucks, et al., 2008). A similar trend
is detected in healthy and diseased tissues in human
(Mahlab, et al., 2012). Consequently, the tRNA
adaptation index (tAI) (dos Reis, et al., 2004) is
applied as a measure for ranking the adaptation of a
gene in term of translation elongation. The
assumption is that the availability of relevant tRNA
types has a strong effect on the efficiency and speed
of translation (Mahlab, et al., 2012; Tuller, et al.,
2010).
Synapses are autonomous structures at nerve
terminals that are specified by high metabolic
demands, and functional plasticity. Communication
across the synapse is mediated by neurotransmitters
(NT) and neuropeptides (NP) that are released from
synaptic vesicles (SV) as a result of neuronal
activity. A success coupling of the action potential to
151
Mahlab S., Linial I. and Linial M..
Translation Efficiency of Synaptic Proteins and Its Coding Sequence Determinants.
DOI: 10.5220/0004238401510157
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2013), pages 151-157
ISBN: 978-989-8565-35-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

exocytosis requires a coordinated action of priming,
targeting and docking (Ferro-Novick and Jahn,
1994). Actually, tens of proteins that belong to SVs
and secretory granules participate in storing,
docking, fusion and recovery in synapses (e.g.,
worm, fly, human) (Broadie and Richmond, 2002).
The intense energetic demand is maximized (Ames,
1992) upon extensive brain activity and experience,
which is the basis for synapse plasticity (Nestler,
2001).
Most synaptic proteins belong to the secretory
systems. From a cellular perspective, the main sub-
compartments include: (i) the trafficking organelles
such as the Endoplasmic Reticulum (ER), Golgi,
endosomes, lysosomes and secretory granules. (ii)
The plasma membrane (PM) with a partition to pre-
and post-synaptic sites. (iii) The extracellular space
and the synaptic cleft.
Neurons are unique with respect to their ability
for local translation (Martin, et al., 2000). A tight
regulation of translation is achieved by translation
inhibition (Richter and Sonenberg, 2005). Still, 5-
10% of the brain transcripts have the potential for a
local translation at synaptic sites (Gebauer and
Hentze, 2004).
Misfolding of proteins in neurons is the basis for
diseases such as Prion, Alzheimer’s (AD) and
Parkinson’s diseases (PD) (Chiti and Dobson, 2006).
Other conditions with memory loss are associated
with a failure in the balance of synaptic proteins and
their proper folding (Ross and Poirier, 2004).
Unlike vesicle trafficking, the SV fusion in
synaptic structures is tightly regulated in time and
space (Brachya, et al., 2006; Trimble, et al., 1991).
The synaptic protein catalogue (Pielot, et al., 2012;
Yanay, et al., 2008) allows testing the evolutionary
refinement on the translational capacity. In this
study, we examine whether the synapse homeostasis
is governed by managing a stable production of
proteins at the right quantities. We propose a
translational dependent strategy to handle the
extreme metabolic and proteomic demands of
synaptic proteins across model organisms.
In this study, we address the notion of sequence-
encoded component of ‘speed controls’ as shaped by
evolution. We hypothesized that the sequences of
synaptic proteins, especially those needed at high
amounts or under restricted conditions are prone to
production failure. We will not elaborate on
additional critical factors that directly alter
translation elongation such as mRNA folding or
translational initiation (Holcik, et al., 2000).
2 METHODS
2.1 Databases
We retrieved sequences from UniProtKB according
to the selected organisms and their subcellular
localization annotations (Barrell, et al., 2009). We
applied the terms “synapse”, “pre-synaptic” and
“post-synaptic” and “complete proteome”. We
extracted proteins that are marked as ‘fragment’. A
partition of proteins to non-disjoint groups was
performed using the UniProtKB Sequence Features
(FT). We tested features such as ‘Signal peptide’,
‘Transmembrane’ (TMD), ‘Disulfide-bond’ and
‘Coiled coil’.
2.2 Computing tAI
tRNA adaptation index (tAI) was computed
according to (dos Reis, et al., 2004). The adaptation
of tRNAs (tAI) is calculated from the genomic
tRNA CN, combined with thermodynamic
considerations of the codon-anticodon interaction.
While the tAI is associated with each codon, the tAI
of a gene is the average of its codons’ tAI. This
measure gauges the availability of tRNAs for each
codon along an mRNA. As codon-anticodon
coupling is not unique due to wobble interactions,
practically, several anticodons can recognize the
same codon, with somewhat different efficiency.
Formally, Let ni be the number of tRNA
isoacceptors recognizing codon i. Let tCGNij be the
copy number of the jth tRNA that recognizes the ith
codon, and let Sij be the selective constraint on the
efficiency of the codon-anticodon coupling. We
define the absolute adaptiveness, Wi, for each codon
i as:
(1)
From Wi we obtain wi, which is the relative
adaptiveness value of codon i, by normalizing the
Wi values (dividing them by the maximal of all 61
Wi).
2.3 Computing Segmental tAI
Local tAI is calculated by dividing each coding
sequence into several overlapping windows (window
of 30, overlapping by 15 codons). For sequences that
are shorter than 180 amino acids, only local
segmental tAI were calculated. This was applied to
Wi (1 Si j )tCGNij
j 1
ni
BIOINFORMATICS2013-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
152

avoid overlap between N’ and C’ terminal windows.
The successive windows are marked N1, N2 and a
similar notation for C’-terminal. In this study we
only focus on the N’-terminal region.
2.4 Statistical Analysis
Statistical significance, correlations, variance and p-
values were according to standard MatLab suite. We
used the t-test and Kolmogorov–Smirnov (KS)-
statistics.
3 RESULTS
3.1 tAI Values in Worm, Fly and
Human
The major model organisms for studying neuronal
functions include human, worm and fly. These
organisms were used to identify the common
molecular apparatus for fast and slow transmission
in the CNS. We took advantage of the manually
compiled SVs and the synaptic proteins catalogue
from human, fly and worm in order to find the
evolutionary signals that impact their translation
efficiency.
Table 1: Correlations of tAI codons in model organisms.
Hs Dm Ce
H. sapiens 1 0.57 0.46
D. melanogaster 1 0.6
C. elegans 1
The tRNAs CN is subjected to evolutionary forces.
Thus, it is significantly different along the
evolutionary tree. For example, there are 87 tRNAs
in E. coli K12, 287 in S. cerevisiae (budding yeast)
and over 3600 in Bos taurus (cow). The CN for
tRNAs in D. melanogaster (Dm, fruitfly) and C.
elegans (Ce, worm) is 299 and 606, respectively.
The variation in tRNA composition for tRNA
isoacceptors and the fraction for each isoacceptors
from the number of tRNA active genes is converted
to tAI values (dos Reis, et al., 2004) (see Methods).
Table 1 displays the correlation between the tAI
values of 61 codons (excluding stop codons) of the
selected model organisms. For example, for human
and fruit fly it is only 0.57 (p-value=6.7e-7) and the
correlation between C. elegans and D. melanogaster
is 0.6 (p-value=1.9e-7). Thus, the tAI values per se
cannot explain any apparent similarity in translation
signals across these species.
3.2 Global tAI values in synapse
The synapse is an autonomous structure.
Schematically, the synaptic proteins can be assigned
to the following functionalities: (i) SNAREs, the
minimal set of proteins that function in SV docking
and fusion. (ii) Direct regulator of SNAREs (iii) Ion
channels and transporters. (iv) Enzymes and
modifiers (e.g., kinases, phosphatases). (v)
Organizers, mainly PDZ and cytoskeleton proteins.
To test whether the above division is
recapitulated by the calculated tAI values, we
compiled a set of 167 synaptic proteins from C.
elegans as a test case. This relatively small set is
manually curated. For consistency, we maintained
identical annotation protocol throughout the study
(see Methods). The majority of C. elegans synaptic
proteins are channels and transporters (class 1.A.9,
NT receptor, and Ligand-gated ion channel). The
largest group includes >30 LGC (Ligand-gated
channel) proteins. These are the homologs of
vertebrate Glycine receptor superfamily.
Fig. 1 shows the sorted tAI values of the entire
set. About 20% of the genes deviate from the global
tAI mean value by >1 s.d. A segregation of
functional groups with proteins with relatively high
global tAI values was noted (noted as HAT).
Specifically, this fraction in enriched with proteins
that are associated with SV biogenesis, SNAREs and
their direct regulators. The enrichment of low tAI
proteins (LATS) includes ligand gated ion channels
(13 genes, >1 s.d., Enrichment score p-
value=0.0014). None of the ion channel (total 103)
was included in this list of 20 proteins with the
highest tAI values.
We found that extremely low values of global tAI
are associated with ligand gated channels. The
possibility that very high sequence similarity explain
this observation was discard. Actually, the C.
elegans ligand gated channels share only 50%
similarity at the amino acids and less than 40% at
the nucleotide levels. Among the ionotropic NT
receptors that are specified by low global tAI values
are the Acetylcholine (ACh), Glycine, GABA and
Glutamate receptors. Importantly, there is no
difference in view of the tAI between cationic
channels (e.g., AChR) and the anionic channels
(GABA/ Glycine receptors). Note that the overall
topology (i.e., N-terminal region facing the
extracellular / SV lumen space is common to these
channels. A similar distribution in tAI values is
noted for all tested organisms (Fig. 1, human).
TranslationEfficiencyofSynapticProteinsandItsCodingSequenceDeterminants
153
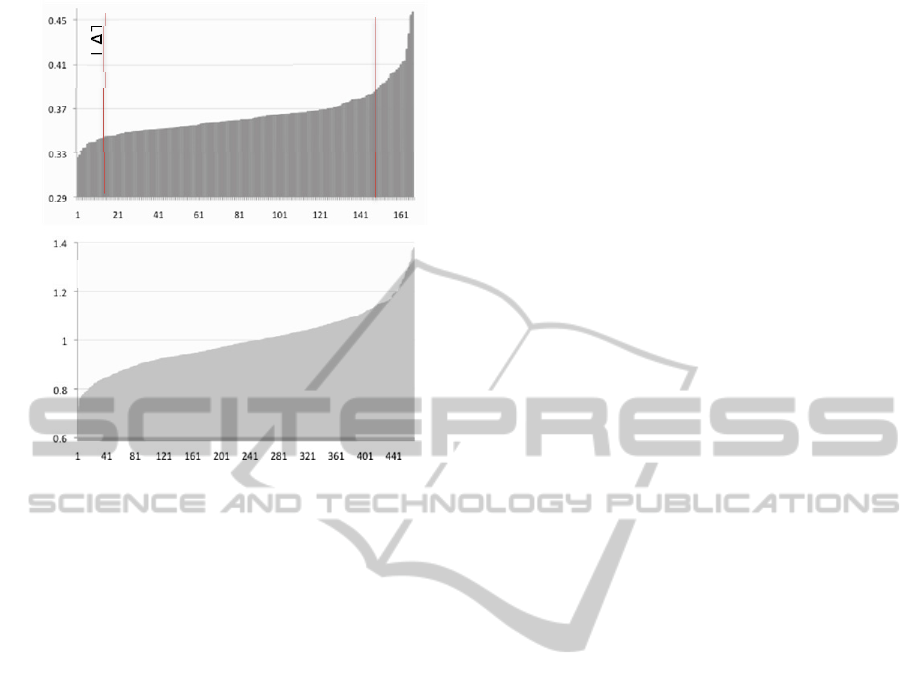
Figura 1: Global tAI of synaptic proteins from C. elegans
(top) and human (bottom). LAT and HAT are proteins that
significantly deviate (>1 s.d.) from the calculated mean
tAI value and are associated with low and high values,
respectively. Relative tAI measures the deviation relative
to the mean which is defined as Relative tAI of 1.0.
3.2.1 tAI Values across Organisms
We repeated the analysis presented above for
synaptic proteins of the fly, worm and human. In
human, we collected 469 synaptic proteins according
to their ‘cellular location’ annotation. A striking
observation is the abundance of membranous
proteins in this set (total of 63%). Some proteins are
associated to the membrane through an indirect
protein-protein interaction (PPI) network, or via a
lipid modification moiety (e.g., GPI anchor).
Functional synapse is strongly dependent on proteins
localization, protein state in term of its modification
and the abundance of proteins. The membranous
proteins in the synapse that contain TMD (single or
multi-pass) comprise the majority of the
membranous synaptic proteins (71% in human). A
large fraction of which includes receptors, ion
channels and transporters that are located to PM. An
additional set comprises proteins that are secreted.
Many of them are short proteins.
We found high correspondence in the list of proteins
that have maximal global tAI values across
organisms. A good example is the Complexin family
(Sudhof and Rothman, 2009). This protein forms a
tight PPI for directly regulating the SNARE complex
formation. As such, it is a major component in
controlling SV exocytosis. Complexins in mammals
are composed of 3 related genes (a single gene in fly
and worm). Multiple sequence alignment for
Complexin from human to chicken and Xenopus
showed that the core of the proteins is highly
conserved (69.4% amino acid identity). More
surprising is the observation that the calculated tAI
is extremely high for all tested organisms and the
calculated tAI value is within the top 15% of the
synaptic proteins (469 in human, 167 in worm and
203 in fly).
The overlap in the list of protein with maximal tAI
along the organisms is very significant. These
proteins are also among the most abundant proteins
of SVs. This set includes the SNAREs (VAMP,
Syntaxin, SNAP-25) as well as synaptotagmin, and
synaptophysin. In addition we noted that key
proteins that participate in endocytosis such as AP-2,
Unc-13 and Endophilin share the property of
extreme tAI value across organisms. This is a highly
significant finding in view of the moderate
correlation in tAI codon values for the tested
organisms (Table 1).
Enrichment tests with respect to GO-Slim
annotations (Barrell, et al., 2009) was performed for
the high and low global tAI values quartiles (the
complete list of synaptic proteins is used as a
background). The enriched terms (p-value <0.01)
for the high tAI include SVs, protein transport,
membrane docking, membrane fusion, synaptic
plasticity. The quartile of the proteins with the
lowest tAI values are enriched with regulation of
small GTPase, and anion transport.
3.3 Local tAI Values in Synapse
The tAI is an indirect measure that affect the
allocation of ribosomes on the transcript. Extreme
values of tAI are associated with ion channels (low),
SNAREs and their primary regulators (high). The
high global tAI of key proteins is in accord of high
production. However, it was proposed that the initial
segment is a critical feature for ribosomal flow
management control.
We evaluate the synaptic proteins in view of the
notion of 'speed controls'. Effectively, it is reflected
by an unequal distribution of low (low adapted
tRNA segments, called LATS) or high tRNA-
adapted codons (HATS). The idea tested extensively
for yeast and E. coli (Tuller, et al., 2010). In
metazoa, the picture is somewhat more complex and
the tAI values strongly correlate with codon usage,
gene expression, protein expression and GC content
(Mahlab, et al., 2012).
Global tAI
HAT
LA
T
Relative tAI
Human
C. elegans
BIOINFORMATICS2013-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
154
We compared the properties of the entire human
proteome and those of the synaptic proteome. Table
2 summarizes the global (the entire coding
sequence) and local (a segment of the coding
sequence) measure of synaptic protein (tAI-local,
coined TAIL). The calculated TAIL for the first
segment of the coding sequence (N1-TAIL) is
reported.
For synaptic proteins we noted that N1-TAIL tAI
value is statistically lower than that of the rest of the
coding gene, for the tested model organisms.
Notably, the average tAI value for all synaptic
proteins was used as a reference. Thus, the inherent
bias for the synaptic proteins was avoided.
The cytosolic fraction in the human proteome
occupies about 70% of all proteins (Fig. 2A, 11,500
proteins). We repeated the test for the local property
to the entire human proteome (TAIL, tAI of a
selected window).
The cytosolic fraction in the human proteome
occupies about 70% of all proteins (Fig. 2A, 11,500
proteins). We repeated the test for the local property
to the entire human proteome (TAIL, tAI of a
selected window).
Table 2: A global measure of tAI for synaptic protein.
#Syn
a
#Proteins
Syn
tAI
SynN1
TAIL
P‐value
TAIL/tAI
Hs 469 18,433
0.329
0.328 0.045
Ce 167 3187
0.364
0.348 3.42E‐8
Dm 203 3094
0.329
0.312 1.96E‐9
a
N1, TAIL for the 30-codon segment of the N-terminal. Syn, synaptic
proteins; Hs, Ce and Dm are the proteomes from human, worm and fly,
respectively.
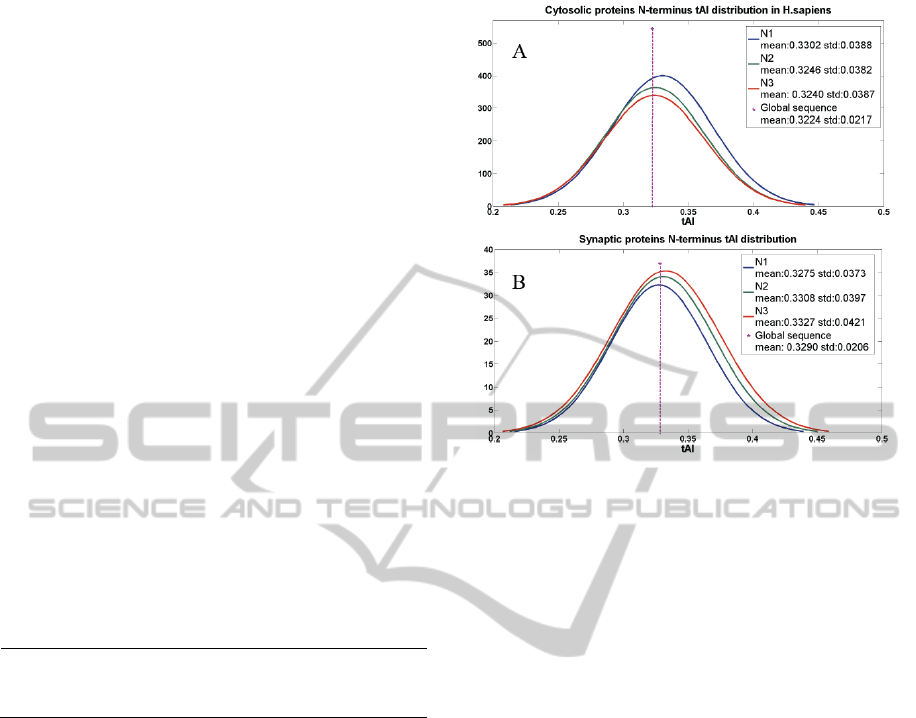
Fig. 2A shows the distribution of the TAIL values
for cytosolic human proteins relative to the same
analysis for the synaptic proteome (Fig. 2B).
Plotting the results for N1, N2 and N3 (each
sequential segment is 30 codons, no overlap)
indicates that the N1 TAIL is higher than the
following segments (N1>N2≥N3). Thus, the human
proteome is signifies by a N1 that has a tendency for
tRNA-adapted codons relative to the tAI of these
genes. However, for the Synaptic proteins, the
opposite trend holds with a calculated TAIL values
in which N1<N2<N3. While the synaptic proteins
display higher global tAI values (vertical dashed
line, Fig. 2), the N1-segment uses codons that are
slightly less adapted. The statistical analysis between
the two sets (Fig. 2) reveals that the variance in the
values of the synaptic proteome is considerably
lower than that of the entire human proteome (mean,
median and statistical error).
Figura 2: TAIL values of human cytosolic and synaptic
proteomes. (A) Distribution of the TAIL for N1, N2 and
N3. Each window covers 30 non-overlapping codons. (B)
Distribution of the TAIL for N1, N2 and N3 for synaptic
proteins. The global tAI is marked as a dashed line. The x-
axis range is identical for Fig. 2A and 2B.
4 DISCUSSION
Based on the results from this study, we test whether
the synaptic proteome is uniquely marked with
translation ‘speed controls’ signals. Several
properties signify synaptic proteins: the high
tendency of membranous proteins, diverse
cytosleletal proteins, precise subcellular localization
and accurate allocation of proteins to organelles (e.g.
SVs, endosomes, recycling vesicles). Many of the
proteins of the synapse share properties such as the
abundance of disulfide bonds, coiled-coil and
membrane association.
The SV is an autonomous organelle that accounts
for a substantial fraction of the protein mass in the
synapse. For example, synaptophysin and VAMP
together, account for 5% of all synaptic proteins.
The SV anatomy supports such load. A pre-synaptic
structure in the human or mouse hippocampus
contains about 300 SVs. Each of the SVs is
composed of tens of proteins. The quantitative
composition of the SVs was revisited using mass
spectrometry (Takamori, et al., 2006) and
quantitative antibodies imaging tools (Mutch, et al.,
2011). Each of the key proteins (VAMP, Rab3, SV2
and Synaptophysin) appears with 5-30 copies per
TranslationEfficiencyofSynapticProteinsandItsCodingSequenceDeterminants
155

SV. Consequently, the mass and protein packing in
SV is maximal.
5 CONCEPTUAL REMARKS
We postulate that variants in N1-TAIL are attractive
to cope with changing metabolic and activity status
of the synapse.
We suggest that in addition to the regulation for
uORFs (upstream ORFs) and activation of
alternative splicing, the translation regulation is an
additional mode for fine-tuning the overall protein
production. Investigating translational signals along
the transcripts of synapse is only in its infancy. We
expect methods such as ribosomal profiling (Ingolia,
et al., 2009) to provide quantitative data for
translation speed and efficiency.
An open question that we began to explore
challenges the translational management with
respect to ribosomal subunits in dendrites (and other
neuronal compartment) (Sutton and Schuman,
2006). We postulate that local translation is critical
for fast and efficient translation under conditions of
restricted resources. Recently, it was shown that
hundreds of mRNAs are localized to neuronal
compartments such as synaptic neuropils (Cajigas, et
al., 2012). Translation of such mRNA must be
highly regulated at the levels of transcript
accessibility and the translation efficiency. Here, we
provide a glimpse on an overlooked evolutionary
encoded signal for managing translation of synaptic
proteins.
ACKNOWLEDGEMENTS
We thank Amos Stern for useful discussions. The
work is supported by Prospects EU FRVII
consortium.
REFERENCES
Ames, A., 3rd (1992) Energy requirements of CNS cells as
related to their function and to their vulnerability to
ischemia: a commentary based on studies on retina,
Can J Physiol Pharmacol, 70 Suppl, S158-164.
Arava, Y., et al. (2003) Genome-wide analysis of mRNA
translation profiles in Saccharomyces cerevisiae, Proc
Natl Acad Sci U S A, 100, 3889-3894.
Barrell, D., et al. (2009) The GOA database in 2009-an
integrated Gene Ontology Annotation resource,
Nucleic Acids Res, 37, D396-403.
Brachya, G., et al. (2006) Synaptic proteins as multi-
sensor devices of neurotransmission, BMC Neurosci,
7 Suppl 1, S4.
Broadie, K.S. and Richmond, J.E. (2002) Establishing and
sculpting the synapse in Drosophila and C. elegans,
Curr Opin Neurobiol, 12, 491-498.
Cajigas, I.J., et al. (2012) The local transcriptome in the
synaptic neuropil revealed by deep sequencing and
high-resolution imaging, Neuron, 74, 453-466.
Chiti, F. and Dobson, C.M. (2006) Protein misfolding,
functional amyloid, and human disease, Annu Rev
Biochem, 75, 333-366.
dos Reis, M., et al. (2004) Solving the riddle of codon
usage preferences: a test for translational selection,
Nucleic Acids Res, 32, 5036-5044.
Ferro-Novick, S. and Jahn, R. (1994) Vesicle fusion from
yeast to man, Nature, 370, 191-193.
Gebauer, F. and Hentze, M.W. (2004) Molecular
mechanisms of translational control, Nat Rev Mol Cell
Biol, 5, 827-835.
Gingold, H. and Pilpel, Y. (2011) Determinants of
translation efficiency and accuracy, Mol Syst Biol, 7,
481.
Holcik, M., et al. (2000) Internal ribosome initiation of
translation and the control of cell death, Trends Genet,
16, 469-473.
Ikemura, T. (1985) Codon usage and tRNA content in
unicellular and multicellular organisms, Mol Biol
Evol, 2, 13-34.
Ingolia, N.T., et al. (2009) Genome-wide analysis in vivo
of translation with nucleotide resolution using
ribosome profiling, Science, 324, 218-223.
Lucks, J.B., et al. (2008) Genome landscapes and
bacteriophage codon usage, PLoS Comput Biol, 4,
e1000001.
Mahlab, S., et al. (2012) Conservation of the relative
tRNA composition in healthy and cancerous tissues,
RNA. 18, 640-652.
Marais, G. and Duret, L. (2001) Synonymous codon
usage, accuracy of translation, and gene length in
Caenorhabditis elegans, J Mol Evol, 52, 275-280.
Martin, K.C., et al. (2000) Local protein synthesis and its
role in synapse-specific plasticity, Curr Opin
Neurobiol, 10, 587-592.
Mutch, S.A., et al. (2011) Protein quantification at the
single vesicle level reveals that a subset of synaptic
vesicle proteins are trafficked with high precision, J
Neurosci, 31, 1461-1470.
Nestler, E.J. (2001) Molecular basis of long-term plasticity
underlying addiction, Nat Rev Neurosci, 2, 119-128.
Percudani, R. (2001) Restricted wobble rules for
eukaryotic genomes, Trends Genet, 17, 133-135.
Pielot, R., et al. (2012) SynProt: A Database for Proteins
of Detergent-Resistant Synaptic Protein Preparations,
Front Synaptic Neurosci, 4, 1.
Plotkin, J.B. and Kudla, G. (2010) Synonymous but not
the same: the causes and consequences of codon bias,
Nat Rev Genet, 12, 32-42.
Richter, J.D. and Sonenberg, N. (2005) Regulation of cap-
dependent translation by eIF4E inhibitory proteins,
BIOINFORMATICS2013-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
156

Nature, 433, 477-480.
Ross, C.A. and Poirier, M.A. (2004) Protein aggregation
and neurodegenerative disease, Nat Med, 10 Suppl,
S10-17.
Sharp, P.M., et al. (1993) Codon usage: mutational bias,
translational selection, or both? Biochem Soc Trans,
21, 835-841.
Sudhof, T.C. and Rothman, J.E. (2009) Membrane fusion:
grappling with SNARE and SM proteins, Science,
323, 474-477.
Sutton, M.A. and Schuman, E.M. (2006) Dendritic protein
synthesis, synaptic plasticity, and memory, Cell, 127,
49-58.
Takamori, S., et al. (2006) Molecular anatomy of a
trafficking organelle, Cell, 127, 831-846.
Trimble, W.S., et al. (1991) Cellular and molecular
biology of the presynaptic nerve terminal, Annu Rev
Neurosci, 14, 93-122.
Tuller, T., et al. (2010) An evolutionarily conserved
mechanism for controlling the efficiency of protein
translation, Cell, 141, 344-354.
Yanay, C., et al. (2008) Evolution of insect proteomes:
insights into synapse organization and synaptic vesicle
life cycle, Genome Biol, 9, R27.
Zhang, Z., et al. (2010) Nonsense-mediated decay targets
have multiple sequence-related features that can
inhibit translation, Mol Syst Biol, 6, 442.
TranslationEfficiencyofSynapticProteinsandItsCodingSequenceDeterminants
157
