
Comparison of Four Ab Initio MicroRNA Prediction Tools
Müşerref Duygu Saçar and Jens Allmer
Molecular Biology and Genetics, Izmir Institute of Technology,Urla, Izmir, Turkey
Keywords: miRNA, Ab Initio, miRNA Prediction, Comparison.
Abstract: MicroRNAs are small RNA sequences of 18-24 nucleotides in length, which serve as templates to drive post
transcriptional gene silencing. The canonical microRNA pathway starts with transcription from DNA and is
followed by processing by the Microprocessor complex, yielding a hairpin structure. This is then exported
into the cytosol where it is processed by Dicer and next incorporated into the RNA induced silencing
complex. All of these biogenesis steps add to the overall specificity of miRNA production and effect.
Unfortunately, experimental detection of miRNAs is cumbersome and therefore computational tools are
necessary. Homology-based miRNA prediction tools are limited by fast miRNA evolution and by the fact
that they are template driven. Ab initio miRNA prediction methods have been proposed but they have not
been analyzed competitively so that their relative performance is largely unknown. Here we implement the
features proposed in four miRNA ab initio studies and evaluate them on two data sets. Using the features
described in Bentwich 2008 leads to the highest accuracy but still does not provide enough confidence into
the results to warrant experimental validation of all predictions in a larger genome like the human genome.
1 INTRODUCTION
MicroRNAs (miRNAs) are a group of small
noncoding RNAs, discovered in the early 90s by
Ambrost and colleagues (Lee et al., 1993), which
convey posttranscriptional regulation. In most cases
miRNAs lead to down regulation of their target
mRNAs but translational activation has also been
observed (Ørom et al., 2008). It has been estimated
that 60% of all human genes are regulated by
miRNAs (Friedman et al., 2009). Another estimate
is that there are more than 1000 miRNAs in the
human genome (Berezikov et al., 2005). MiRNAs
can come from introns (Morlando et al., 2008),
coding regions (Rodriguez et al., 2004), or
intergenic miRNA gene clusters (Altuvia et al.,
2005). It has been suggested that a miRNA may
regulate hundreds of targets (Enright et al., 2003).
MiRNAs can, therefore, form complex regulatory
networks. Not surprisingly, miRNAs are implicated
in diseases such as cardiovascular disease (Elton et
al., 2011) and cancer (Suzuki and Miyazono, 2011).
The biogenesis of miRNAs follows largely the
canonical pathway. Initially, DNA is transcribed into
RNA by either RNA polymerase II (Lee et al., 2004)
or III (Borchert et al., 2006) and then the
microprocessor complex (Han et al., 2006) cleaves
hairpin structures from the transcript. These hairpins
are exported into the cytosol by Exportin 5 (Lund et
al., 2004; Zeng and Cullen, 2004; Okada et al.,
2009) where they are cleaved by Dicer (Cifuentes et
al., 2010) and then loaded onto the RNA induced
silencing complex
Despite the great effort that has been put into the
elucidation of the miRNA pathway, not much is
known which would facilitate computational
modeling that is based on clear processing facts
instead of data mining approaches. Two approaches
are available for miRNA prediction, one based on
homology and the other free of any references
named ab initio.
While homology-based methods seem straight
forward, they only retrieve results similar to already
known miRNAs and rarely allow the detection of
novel miRNAs (Bentwich et al., 2005). Furthermore,
miRNA evolution progresses at a high rate (Liang
and Li, 2009); (Lu et al., 2008), which limits the
applicability of homology-based methods.
Ab initio prediction methods (Ng and Mishra,
2007); (Lai et al., 2003); (Bentwich, 2008); (Ding et
al., 2010); (Jiang et al., 2007); (Pfeffer et al., 2005);
(Xue et al., 2005); (Yousef et al., 2006); (Grundhoff,
2011); (Burgt et al., 2012); (Cakir and Allmer,
2010); (Ritchie et al., 2012) try to extract parameters
which describe hairpin structures, an element which
190
Saçar M. and Allmer J..
Comparison of Four Ab Initio MicroRNA Prediction Tools.
DOI: 10.5220/0004248201900195
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2013), pages 190-195
ISBN: 978-989-8565-35-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

is deemed important in the miRNA genesis process,
and uses these features for machine learning to
detect miRNAs. Although many of the ab inito
algorithms that have been proposed report their
accuracy, they cannot be easily compared as they are
run on different data sets. Furthermore, most of the
algorithms cannot be obtained. Therefore, we
implemented all features described in Ding et al.,
Jiang et al, Ng and Mishra, and Bentwich (Ng and
Mishra, 2007); (Bentwich, 2008); (Ding et al.,
2010); (Jiang et al., 2007) and compared them on the
same data set to investigate relative algorithm
performance. Ding et al. performed best on the data
sets which we prepared but the maximum achieved
accuracy of 0.996 would produce too many false
positives so that experimental validation of all
predictions would not be cost and time effective.
Furthermore, this high accuracy seems to be an
outlier and in all other cases that we tested Bentwich
2008 outperforms Ding et al. 2010 with a maximum
accuracy of 0.986 that is closely reproduced among
data sets. Therefore, we advise the use of the
features described in Bentwich 2008 when
attempting ab initio hairpin prediction.
2 MATERIALS AND METHODS
The four tools which we wanted to compare are not
available as software so we implemented all features
that were proposed in the papers in Java™ and used
our code to calculate the values from the negative
and positive data sets.
2.1 Features
The features that are used to discriminate between a
true miRNA hairpin and a pseudo one are different
among studies, but sometimes the feature is the same
and just the naming differs. In the following we list
the features that were used in the studies compared
here and add the acronym that we gave to the feature
in parentheses. Features are also summarized in
Table 1 for ease of reference.
2.1.1 Features Used in Ng and Mishra 2007
Sequence based features; 16 dinucleotide
frequencies %NN (%AA, %AC, %AG, %AU, %CA,
%CC, %CG, %CU, %GA, %GC, %GG, %GU,
%UA, %UC, %UG, %UU) and 1 aggregate
dinucleotide frequency %G+C (%G++%C).
Probability based features derived from
dinucleotide shuffling (dns); adjusted base pairing
propensity dP (dns_p(bpp), dns_p(bpp/hpl)),
adjusted Minimum Free Energy of folding (MFE)
dG (dns_p(hpmfe_rf), dns_p(hpmfe_rf/hpl)), MFE
index 1 MFEI1 (hpmfe_rf_I1), adjusted base pair
distance dD (dns_p(bpd), dns_p(bpd/hpl)), adjusted
shannon entropy dQ (dns_p(Q), dns_p(Q/hpl)),MFE
index 2 MFEI2 (hpmfe_rf/ns), degree of
compactness dF (dc) and normalized variants of dP,
dG, dQ, dD and dF (dns_z(bpp), dns_z(bpp/hpl),
dns_z(hpmfe_rf), dns_z(hpmfe_rf/hpl), dns_z(Q),
dns_z(bpd/hpl), dns_z(bpd), dns_z(Q/hpl)).
2.1.2 Features Used in Jiang et al., 2007
Structural features; 32 triplet elements i.e. U(((’,
‘A((.’, etc. [*U(((, *U..., *U.((, *U(.., *U(.(, *U..(,
*U..., *U.(., *C(((, *C(.(, *C..(, *C((., *C.(., *C(..,
*C.((, *C..., *A(((, *A..., *A(.., *A(.(, *A.(., *A..(,
*A.((, *A((., *G(((, *G(.(, *G((., *G(.., *G.((, *G.(.,
*G..(, *G...)], mfe (hpmfe_rf/ns) and P-value
(dns_p(hpmfe_rf)).
2.1.3 Features Used in Bentwich, 2008
Structural features; hairpin length (hpl), loop length
(hll), free energy per nucleotide (hpmfe_rf/hpl),
matching base pairs (bpp) and maximal bulge size
(mbs).
Sequence based features; abundance of any
dinucleotide, AA, AT, etc. (#AA, #AC, #AG, #AU,
#CA, #CC, #CG, #CU, #GA, #GC, #GG, #GU,
#UA, #UC, #UG, #UU), regular internal repeat (dr),
inverted internal repeat (ir), free energy (hpmfe_rf)
and GC content (%GC).
2.1.4 Features Used in Ding et al., 2010
Structural features; triplet elements A(((, A…, U(((,
U(.(, U…, G(((, C(((, C(.(, [*A(((, *A…, *U(((,
*U(.(, *U…, *G(((, *C(((, *C(.(].
Sequence based features; base pairing propensity
dP (bpp), dP/n_loops (bpp/nl), Avg_bp_stem
(bpp/sl), diversity (bpd), |A-U|/L (%AU), |G-C|/L
(%GC), %(A-U)/n_loops (st(A-U)/ns), %(G-
C)/n_loops (st(G-C)/ns).
Thermodynamics based features; ensemble free
energy NEFE (efe), minimum free energy index 1-4
MFEI1 (hpmfe_rf_I1), MFEI2 (hpmfe_rf/ns),
MFEI3 (hpmfe_rf/ns/hpl), MFEI4 (hpmfe_rf/hpl),
dG (dG), Diff (dme), ensemble frequency Freq (efq),
melting temperature Tm (Tm), enthalpy divided by
length dH/L (dH/hpl), entropy divided by length
dS/L (dS/hpl), Tm/L (Tm/hpl), p-value_MFE
(dns_p(hpmfe_rf)), p-value_EFE (dns_p(efe)), z-
core_MFE (dns_z(hpmfe_rf)), z-score_EFE
(dns_z(efe)).
ComparisonofFourAbInitioMicroRNAPredictionTools
191
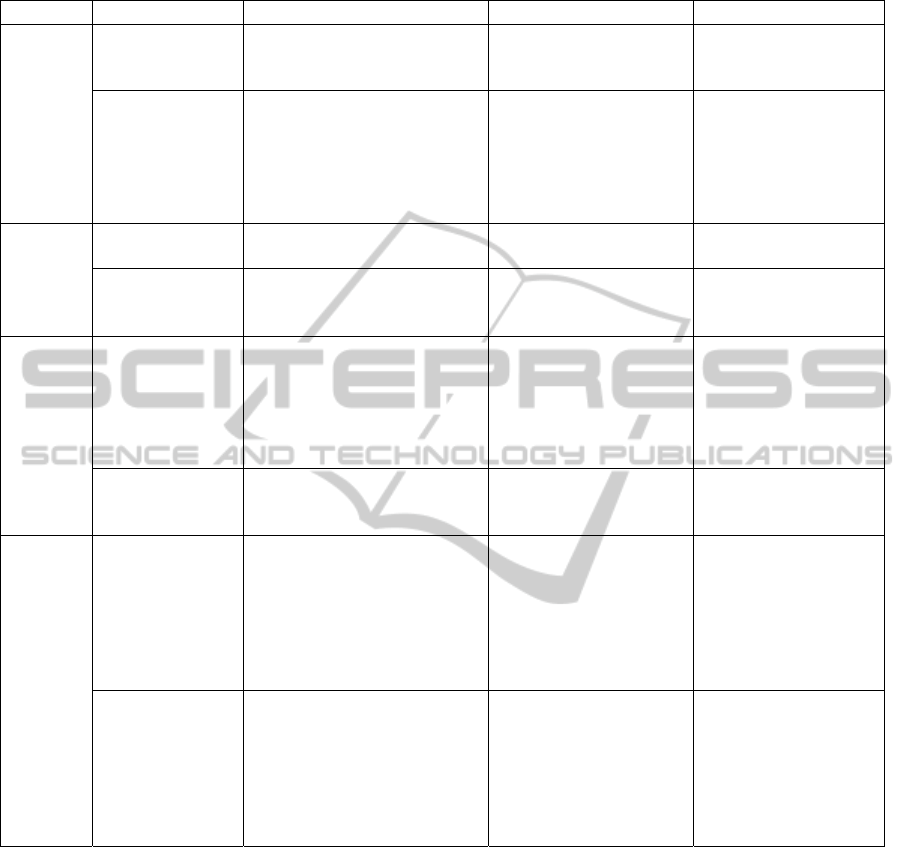
Table 1: Features that were proposed in the selected studies are presented in the first row of the respective study and the
acronyms we chose for those features are presented in the following row.
Studies Sequence-Based Probability-Based Structural Thermodynamic-Based
Ng and
Mishra
2007
16 dfs %NN and 1
aggregate df %G+C
ratio
dP, dG, MFEI1, dD, dQ, MFEI2,
dF, normalized variants of dP,
dG, dQ, dD and dF
%AA-%UU,
%G+C
dns_p(bpp, bpp/hpl, hpmfe_rf,
hpmfe_rf/hpl, bpd, bpd/hpl, Q,
Q/hpl), dns_z(bpp, bpp/hpl,
hpmfe_rf, hpmfe_rf/hpl, bpd,
bpd/hpl, Q, Q/hpl), hpmfe_rf_I1,
hpmfe_rf/ns, dc
Jiang et
al. 2007
32 triplet elements, mfe, P-
value
*A… - *U(((,
(hpmfe_rf/ns),
dns_p(hpmfe_rf)
Bentwich
2008
Dinucleotide
abundance, regular
internal repeats,
inverted internal
repeats, mfe, GC
content
hairpin length, loop length,
free energy per nucleotide,
matching base pairs,
maximal bulge size
#AA - #UU, dr, ir,
(hpmfe_rf), %GC
hpl, hll, (hpmfe_rf/hpl),
bpp, mbs
Ding et
al. 2010
base pairing
propensity (dP),
Avg_bp_stem,
diversity, |A-U|/L,
|G-C|/L, %(A-
U)/n_loops, %(G-
C)/n_loops
triplet elements
NEFE, MFEI1, MFEI2,
MFEI3, MFEI4, dG,
Freq, Tm, dH/L, dS/L,
Tm/L, p-value_MFE, p-
value_EFE, z-
score_MFE, z-
score_EFE
bpp, (bpp/nl),
(bpp/sl), bpd,
%AU, %GC, (st(A-
U)/ns), (st(G-C)/ns)
*A(((, *A…, *U(((, *U(.(,
*U…, *G(((, *C(((, *C(.(
efe, hpmfe_rf_I1,
hpmfe_rf/ns,
hpmfe_rf/ns/hpl, dG,
dme, efq, Tm, dH/hpl,
dS/hpl, Tm/hpl,
dns_p(hpmfe_rf, efe),
dns_z(hpmfe_rf, efe)
2.2 Data Sets
2.2.1 Positive Data Set
MirBase is the de facto standard repository for
miRNAs (Griffiths-Jones et al., 2008). It contains
about 1500 entries for human counting both guide
and passenger strands. We downloaded all human
miRNAs as positive data. From the entries we
removed the ones that contain more than one hairpin
when folded by RNAFold (Hofacker, 2003) or
RNAShapes (Steffen et al., 2006). If no proper link
to Ensemble could be established, the entries were
removed as well. From the remaining, about 1000,
miRNA examples we created five random subsets
containing 500 positive examples each.
2.2.2 Negative Data Sets
Negative data sets are especially difficult to establish
for miRNAs, experimentally or computationally
(Ding et al., 2010); (Ritchie et al., 2012); (Wu et al.,
2011); (Yousef et al., 2008). Since most machine
learning approaches that have been proposed for ab
initio miRNA prediction are built on two class
classification we designed one data set which
consists of random sequences of the same length as
the selected miRNAs in the positive data set. We
BIOINFORMATICS2013-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
192
consider this data set to be easy to solve. Another
data set, the pseudo miRNA data set, was taken from
the Ng and Mishra study and we consider it to be
more difficult (Ng and Mishra, 2007).
2.3 Machine Learning
We created five combined data sets consisting of
60% training and 40% test data from the overall data
set. These data sets were used to train and test SVM
classification using default settings in Orange
Canvas (http://orange.biolab.si/). For performance
evaluation test on ‘test data’ was used. This
approach was used since fivefold cross validation
could not be used with multiple studies at the same
time; but had to be repeated individually, thus
leading to different data sets and therefore to a
potentially unfair comparison.
3 RESULTS AND DISCUSSION
In our opinion, the two datasets that were used (see
Data Sets) are of different difficulty with the random
dataset being easier to solve than the pseudo miRNA
data set. This can also be deduced from the best
results reported in Tables 2 and 3. The results for the
random miRNAs in Table 2 lead to higher accuracy
than the data in Table 3 which is achieved with
pseudo miRNAs. For both tables the best and the
average accuracy are provided along with the
standard deviation, calculated from fivefold cross
validation. Using the features described in the study
by Bentwich 2008 leads to the highest accuracy
without any standard deviation. Judging from these
results it seems trivial to discriminate between true
and false hairpins when using properly selected
features.
Table 2: Accuracy measurements for human miRNAs
(positive dataset) and random miRNAs (negative dataset).
Studies
Accuracy Values
Best Average Standard Deviation
Ng and Mishra 2007 0.919 0.894 0.183
Bentwich 2008 1.000 1.000 0
Ding et al. 2010 1.000 0.676 0.217
Jiang et al. 2007 0.954 0.952 0.003
We did not expect such a perfect result as
achieved by Bentwich 2008 features in Table 2 for
the pseudo miRNAs and Table 3 displays no such
success. For the pseudo miRNA dataset, the features
used in Ding et al. 2010 achieve the highest
accuracy although with a high standard deviation
over the cross validation.
Table 3: Accuracy measurements for human miRNAs
(positive dataset) and pseudo miRNAs (negative dataset).
Studies
Accuracy Values
Best Average Standard Deviation
Ng and Mishra 2007 0.930 0.895 0.060
Bentwich 2008 0.986 0.983 0.002
Ding et al. 2010 0.996 0.599 0.198
Jiang et al. 2007 0.910 0.877 0.018
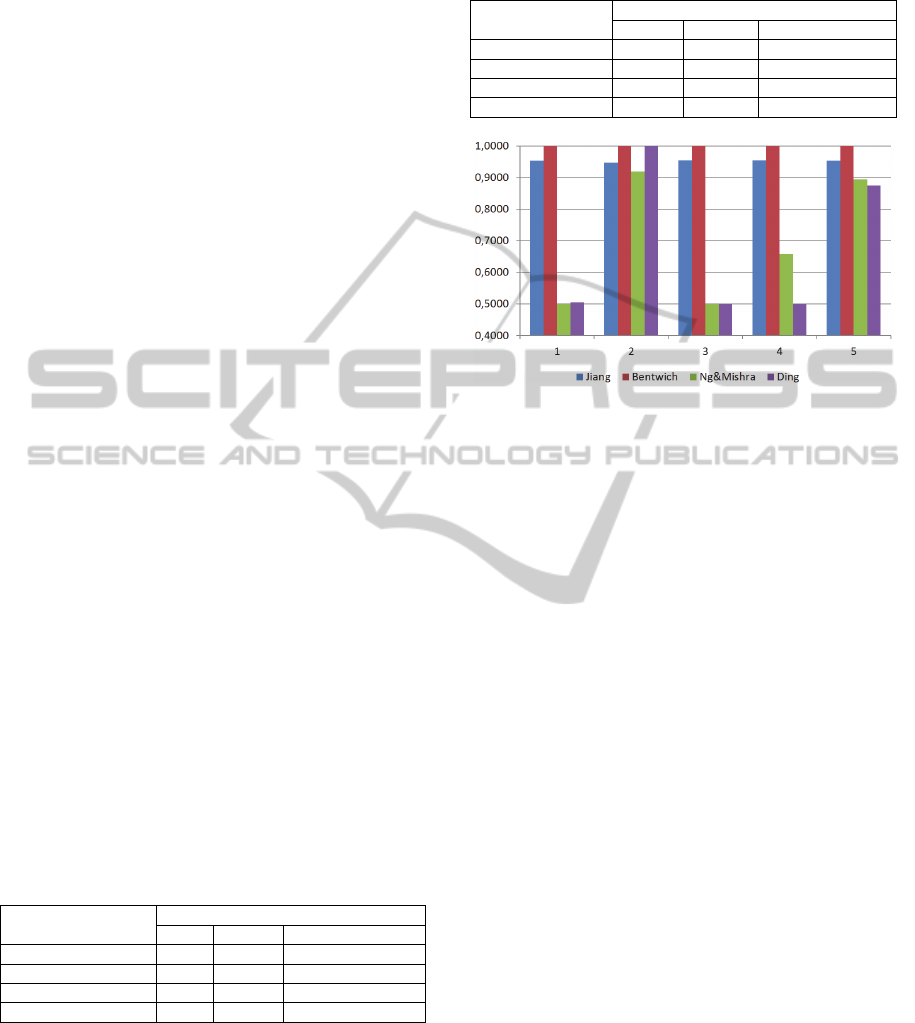
Figure 1: Accuracy measurements for human miRNAs
(positive dataset) and random miRNAs (negative dataset).
All cross validation results are shown individually.
The best accuracy of 0.996, achieved by the
features described in the study by Ding et al. 2010,
when used for a million putative hairpins in human
would lead to 4000 false positive identifications.
Unfortunately, the number of putative hairpins in
human is large and the accuracy calculated here does
not fully reflect the true accuracy.
This is due to the fact that it is not entirely
known what differentiates a true from a false
hairpin. A much higher false positive rate must
therefore be expected for real data and thus the
number of false positives may not allow costly
experimental validation of all predicted miRNAs.
Figures 1 and 2 further support this point by
showing that the accuracy strongly depends on the
data set used for training and testing. This can be
deduced from the variation among the accuracies for
the 5 data sets used in the fivefold cross validation.
For the random miRNAs, the variation among
datasets is large for most studies. However,
Bentwich 2008 always achieves perfect separation
and Jiang et al. 2007 achieves a low variation and an
overall good result (Figure 1).
With the pseudo miRNAs the variation is even
more important to be analysed. Although Ding et al.
2010 achieves the highest accuracy in one case, it
fails in all other cases which shows a strong
dependence on the training and test data set and a
poor generalization for the features from that study
(Figure 2). Bentwich 2008 does not have such
generalization problems and outperforms all other
ComparisonofFourAbInitioMicroRNAPredictionTools
193
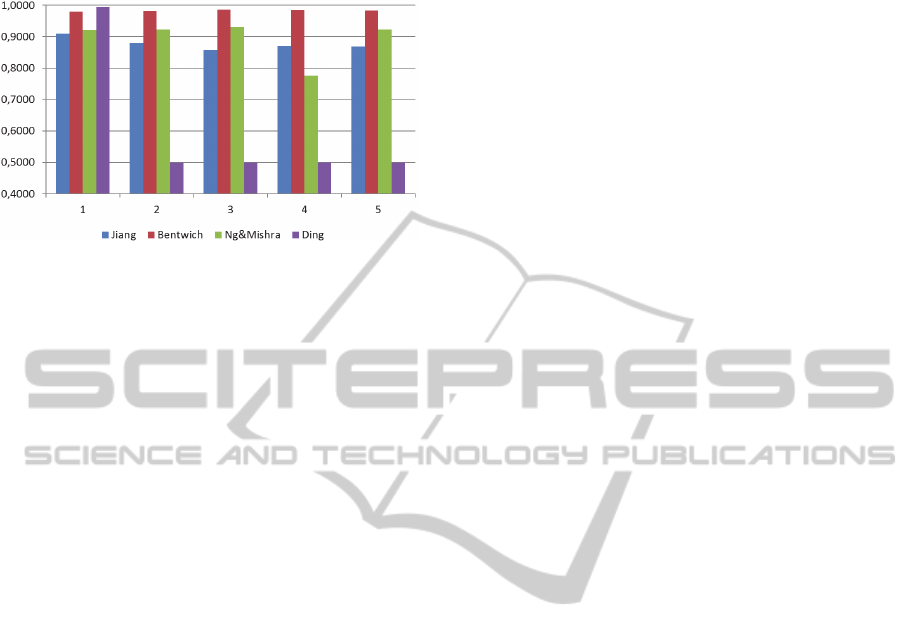
studies on the remaining four data sets.
Figure 2: Accuracy measurements for human miRNAs
(positive dataset) and pseudo miRNAs (negative dataset).
All cross validation results are shown individually.
4 CONCLUSIONS
Although many algorithms have been proposed for
ab initio miRNA gene prediction, they have not been
compared for their relative performance. We
compared four of twelve available ab initio
algorithms in this study and found that Bentwich
2008 achieves the highest accuracy on the random
data set and the second best accuracy on the pseudo
hairpin data set but with a very low variation.
Unfortunately, the achieved accuracy of 0.986
would lead to many false positives which would turn
any attempt at experimental validation of all
predicted miRNAs into a futile endeavor.
In the future, we plan to expand this assessment
of available algorithms to all currently available
ones. We believe it is necessary to establish the
accuracy of existing algorithms not independently
but transparently and comparable. To the best of our
knowledge, this is the first independent assessment
of multiple ab initio miRNA prediction methods.
As negative data sets are hard to come by, we
will try to establish another set of negative data and
further try one-class classification with the proposed
parameters in follow-up studies.
Currently, we advise the use of the features used
in the Bentwich 2008 study when trying ab initio
prediction of hairpins.
ACKNOWLEDGEMENTS
This study was in part supported by an award
received from the Turkish Academy of Sciences
(http://www.tuba.gov.tr) for outstanding young
scientists (TUBA GEBIP).
REFERENCES
Altuvia, Y., Landgraf, P., Lithwick, G., Elefant, N.,
Pfeffer, S., Aravin, A., Brownstein, M. J., Tuschl, T.,
and Margalit, H., 2005. Clustering and conservation
patterns of human microRNAs. Nucleic acids research
33, 2697–706.
Bentwich, I., 2008. Identifying human microRNAs. RNA
interference 320, 257–269.
Bentwich, I., Avniel, A., Karov, Y., Aharonov, R., Gilad,
S., Barad, O., Barzilai, A., Einat, P., Einav, U., Meiri,
E., et al., 2005. Identification of hundreds of
conserved and nonconserved human microRNAs.
Nature genetics 37, 766–70.
Berezikov, E., Guryev, V., van de Belt, J., Wienholds, E.,
Plasterk, R. H. A., and Cuppen, E., 2005. Phylogenetic
shadowing and computational identification of human
microRNA genes. Cell 120, 21–4.
Borchert, G. M., Lanier, W., and Davidson, B. L., 2006.
RNA polymerase III transcribes human microRNAs.
Nature Structural & Molecular Biology 13, 1097–
1101.
Burgt, A. V. D., Fiers, M. W. J. E., Nap, J., and Van, R. C.
H. J., 2012. In silico miRNA prediction in metazoan
genomes : balancing between sensitivity and
specificity. BMC Genomics 24, 1–24.
Cakir, M. V., and Allmer, J., 2010. Systematic
computational analysis of potential RNAi regulation in
Toxoplasma gondii. in Health Informatics and
Bioinformatics, HIBIT), 2010 5th International
Symposium on, Ankara, Turkey: IEEE), 31–38.
Cifuentes, D., Xue, H., Taylor, D. W., Patnode, H.,
Mishima, Y., Cheloufi, S., Ma, E., Mane, S., Hannon,
G. J., Lawson, N. D., et al., 2010. A novel miRNA
processing pathway independent of Dicer requires
Argonaute2 catalytic activity. Science 328, 1694–
1698.
Ding, J., Zhou, S., and Guan, J., 2010. MiRenSVM:
towards better prediction of microRNA precursors
using an ensemble SVM classifier with multi-loop
features. BMC Bioinformatics 11 Suppl 1, S11.
Elton, T. S., Martin, M. M., Sansom, S. E., Belevych, A.
E., Györke, S., and Terentyev, D., 2011. miRNAs got
rhythm. Life Sciences 88, 373–383.
Enright, A. J., John, B., Gaul, U., Tuschl, T., Sander, C.,
and Marks, D. S., 2003. MicroRNA targets in
Drosophila. Genome Biology 5, R1.
Friedman, R. C., Farh, K. K.-H., Burge, C. B., and Bartel,
D. P., 2009. Most mammalian mRNAs are conserved
targets of microRNAs. Genome Research 19, 92–105.
Griffiths-Jones, S., Saini, H. K., van Dongen, S., and
Enright, A. J., 2008. miRBase: tools for microRNA
genomics. Nucleic acids research 36, D154–8.
Grundhoff, A., 2011. Computational prediction of viral
miRNAs. Methods in Molecular Biology, Clifton,
N.J.) 721, 143–152.
BIOINFORMATICS2013-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
194

Han, J., Lee, Y., Yeom, K.-H., Nam, J.-W., Heo, I., Rhee,
J.-K., Sohn, S. Y., Cho, Y., Zhang, B.-T., and Kim, V.
N., 2006. Molecular basis for the recognition of
primary microRNAs by the Drosha-DGCR8 complex.
Cell 125, 887–901.
Hofacker, I. L., 2003. Vienna RNA secondary structure
server. Nucleic Acids Research 31, 3429–3431.
Jiang, P., Wu, H., Wang, W., Ma, W., Sun, X., and Lu, Z.,
2007. MiPred: classification of real and pseudo
microRNA precursors using random forest prediction
model with combined features. Nucleic Acids
Research 35, W339–344.
Lai, E. C., Tomancak, P., Williams, R. W., and Rubin, G.
M., 2003. Computational identification of Drosophila
microRNA genes. Genome Biol 4, R42.
Lee, R. C., Feinbaum, R. L., and Ambrost, V., 1993. The
C . elegans Heterochronic Gene lin-4 Encodes Small
RNAs with Antisense Complementarity to & II-14.
Cell 75, 843–854.
Lee, Y., Kim, M., Han, J., Yeom, K.-H., Lee, S., Baek, S.
H., and Kim, V. N., 2004. MicroRNA genes are
transcribed by RNA polymerase II. The EMBO
Journal 23, 4051–4060.
Liang, H., and Li, W.-H., 2009. Lowly expressed human
microRNA genes evolve rapidly. Molecular biology
and evolution 26, 1195–8.
Lu, J., Shen, Y., Wu, Q., Kumar, S., He, B., Shi, S.,
Carthew, R. W., Wang, S. M., and Wu, C.-I., 2008.
The birth and death of microRNA genes in
Drosophila. Nature genetics 40, 351–5.
Lund, E., Güttinger, S., Calado, A., Dahlberg, J. E., and
Kutay, U., 2004. Nuclear export of microRNA
precursors. Science 303, 95–8.
Morlando, M., Ballarino, M., Gromak, N., Pagano, F.,
Bozzoni, I., and Proudfoot, N. J., 2008. Primary
microRNA transcripts are processed co-
transcriptionally. Nature Structural & Molecular
Biology 15.
Ng, K. L. S., and Mishra, S. K., 2007. De novo SVM
classification of precursor microRNAs from genomic
pseudo hairpins using global and intrinsic folding
measures. Bioinformatics 23, 1321–30.
Okada, C., Yamashita, E., Lee, S. J., Shibata, S., Katahira,
J., Nakagawa, A., Yoneda, Y., and Tsukihara, T.,
2009. A high-resolution structure of the pre-
microRNA nuclear export machinery. Science 326,
1275–1279.
Pfeffer, S., Sewer, A., Lagos-Quintana, M., Sheridan, R.,
Sander, C., Grässer, F. A., van Dyk, L. F., Ho, C. K.,
Shuman, S., Chien, M., et al., 2005. Identification of
microRNAs of the herpesvirus family. Nature
Methods 2, 269–276.
Ritchie, W., Gao, D., and Rasko, J. E. J., 2012. Defining
and providing robust controls for microRNA
prediction. Bioinformatics, Oxford, England) 28,
1058–61.
Rodriguez, A., Griffiths-Jones, S., Ashurst, J. L., and
Bradley, A., 2004. Identification of mammalian
microRNA host genes and transcription units. Genome
Research 14, 1902–1910.
Steffen, P., Voss, B., Rehmsmeier, M., Reeder, J., and
Giegerich, R., 2006. RNAshapes: an integrated RNA
analysis package based on abstract shapes.
Bioinformatics, Oxford, England) 22, 500–3.
Suzuki, H. I., and Miyazono, K., 2011. Emerging
complexity of microRNA generation cascades.
Journal of biochemistry 149, 15–25.
Wu, Y., Wei, B., Liu, H., Li, T., and Rayner, S., 2011.
MiRPara: a SVM-based software tool for prediction of
most probable microRNA coding regions in genome
scale sequences. BMC Bioinformatics 12, 107.
Xue, C., Li, F., He, T., Liu, G.-P., Li, Y., and Zhang, X.,
2005. Classification of real and pseudo microRNA
precursors using local structure-sequence features and
support vector machine. BMC Bioinformatics 6, 310.
Yousef, M., Jung, S., Showe, L. C., and Showe, M. K.,
2008. Learning from positive examples when the
negative class is undetermined--microRNA gene
identification. Algorithms for molecular biology :
AMB 3, 2.
Yousef, M., Nebozhyn, M., Shatkay, H., Kanterakis, S.,
Showe, L. C., and Showe, M. K., 2006. Combining
multi-species genomic data for microRNA
identification using a Naive Bayes classifier.
Bioinformatics, Oxford, England) 22, 1325–34.
Zeng, Y., and Cullen, B. R., 2004. Structural requirements
for pre-microRNA binding and nuclear export by
Exportin 5. Nucleic acids research 32, 4776–85.
Ørom, U. A., Nielsen, F. C., and Lund, A. H., 2008.
MicroRNA-10a binds the 5’UTR of ribosomal protein
mRNAs and enhances their translation. Molecular cell
30, 460–71.
ComparisonofFourAbInitioMicroRNAPredictionTools
195
