
Ex-vivo Platelet Activation using Electric Pulse Stimulation
Nicole LaPlante, V. Bogdan Neculaes, Brian D. Lee, Andrew S. Torres, Kenneth Conway,
Steve Klopman and Antonio Caiafa
GE Global Research Center, Niskayuna, NY, U.S.A.
Keywords: Activated Platelets, Platelet Gel, Wound Healing, Pulsed Electric Field, Growth Factor.
Abstract: Activated platelet rich plasma (PRP), also known as platelet gel, is an encouraging autologous cell therapy
with numerous applications in areas including: wound healing, haemostasis and wound infection control.
Activation of PRP using electric pulse stimulation is a promising alternative to activation via biologics such
as bovine thrombin. By removing the need for biologics, it is possible to deliver a cost-effective, fast, truly
autologous platelet gel option. In this position paper, we describe parameters for effective ex-vivo release of
several growth factors from human platelets in PRP using electric field pulses with the duration of hundreds
of nanoseconds. Growth factor release levels with nanosecond pulse electric fields seem at the same level or
higher compared to bovine thrombin, the standard platelet activator used in clinical practice. These findings
suggest that electric pulse stimulation has the potential to become not only a viable alternative to
biochemical platelet activators, but to actually enhance the desired in vivo biological effects, such as wound
healing.
1 INTRODUCTION
Whole blood contains several components, including
red blood cells, white blood cells, plasma and
platelets. Platelets have a typical lifespan of about
seven to ten days and will concentrate and aggregate
at the site of injury as part of the body’s response to
promote haemostasis, tissue regeneration and
revascularization (Tate and Crane 1999). Platelets
are formed in the bone marrow and contain
populations of granules – such as alpha granules and
dense granules. Normal platelet count in whole
blood is about 200,000 platelets/ul (Tate and Crane,
1999).
Platelet cell therapy is an approach to harvest the
natural ability of the body to stop the bleeding and
promote wound healing. By collecting one’s
platelets, activating them ex-vivo, and placing them
back on the wound, a novel therapeutic approach has
been developed, that dramatically enhances what the
body has been programmed to accomplish naturally.
Platelet gel is a substance containing a
concentrated amount of platelets which are activated
to release proteins found within the alpha granules.
These proteins, which include numerous growth
factors, are released upon platelet activation and
include platelet-derived growth factor (PDGF),
transforming growth factor-beta (TGF- β), vascular
endothelial growth factor (VEGF) and epidermal
growth factor (EGF). Activated platelets, platelet
gel, have been shown to enhance wound healing
(Driver, 2006,, Lacci, 2010), induce hemostasis
(Gunyadin, 2008), and provide antibacterial
protection for the wound as it heals. The application
landscape is quite broad for platelet gel. Among the
many potential clinical applications, effective
therapy has been shown for diabetic foot ulcers,
dentistry, cardiac surgery, cosmetic surgery,
orthopaedic surgery, sports medicine.
Typical workflow for generating platelet gel
performed at the bed side includes a blood draw
from the patient, platelet separation/concentration
(centrifugation is the state of the art method), and,
finally, ex-vivo platelet activation using a
combination of thrombin and calcium chloride. After
activation, platelet concentrates have a gel-like
consistency. The final step is the application of this
gel on the pre-determined wound. Currently, platelet
activation is performed using bovine thrombin (state
of the art in US) or other types of thrombin in
Canada and Europe (recombinant thrombin,
autologous thrombin or human thrombin isolated
from donor plasma). Various types of thrombin
currently used are rather expensive and can have
202
LaPlante N., Bogdan Neculaes V., D. Lee B., S. Torres A., Conway K., Klopman S. and Caiafa A..
Ex-vivo Platelet Activation using Electric Pulse Stimulation.
DOI: 10.5220/0004328602020208
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 202-208
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

significant side effects. For example, bovine
thrombin–associated immune-mediated
coagulopathy will incur a cost per patient from $
16,584 to $ 163,072 (Alexander, 2009). The
workflow of generating autologous thrombin can be
complex and lengthy, about 40 minutes
(http://www.biomet.com/biologics/international/prin
t/BBI0004.1_081508.pdf). Human thrombin from
donor plasma carries a risk for transmitting of
infection diseases (Cada, 2008). A truly autologous,
fast and significantly less expensive platelet
activation method would eliminate potential side
effects and will lower the cost for the platelet gel
treatment, opening opportunities for increased
access of this therapeutic approach.
This autologous, non-animal derived, non-
biochemical activation method would allow a fast
and completely autologous platelet gel solution in
the clinic, for difficult to heal wounds. Autologous
platelet gel could become the standard of care for
difficult to heal wounds, such as diabetic foot ulcers.
2 PLATELET ACTIVATION
USING ELECTRIC PULSE
STIMULATION
Short duration pulse electric fields, in the
nanosecond range, have been shown to have
significant effects on the intracellular structures
(Beebe, 2005). Typically, long pulse electric fields,
larger than 0.1 ms, have been efficiently utilized for
cell membrane permeabilization termed
“electroporation”, with a variety of applications such
as exogenous molecule delivery, transfection/gene
delivery, and tumor cell death using irreversible
electroporation. The use of modelling tools in the
context of an electrical model for biological cells,
predicts that pulses with duration shorter than the
charging time of the outer cellular membrane, can
affect the intracellular organelles (Beebe, 2005).
Experiments have indeed confirmed this
theoretical prediction; numerous experimental
demonstrations have pointed out biological effects
of electric pulse stimulation in the nanosecond
range: modulation of caspase activity (caspases
modulate a variety of cell functions – proliferation,
differentiation, cell cycle), apopotosis – programmed
cell death and calcium mobilization (Beebe, 2005).
These short, nanosecond electric field pulses are
thought to create small pores – nanopores- in the
organelle membranes.
Nanosecond pulse electric field effects on
calcium transport have been recently introduced as a
novel method for ex-vivo platelet activation (Zhang,
2008). It has been hypothesized that nanosecond
pulse electric fields, nsPEF, cause calcium to leak
out from the intracellular stores as of result of
nanopores being created in organelles membrane, as
well as an influx of extracellular calcium through
plasma membrane nanopores (Zhang, 2008). This
calcium transport with nsPEF has been correlated
with platelet activation; initial experiments using
newly outdated platelets from the American Red
Cross showed platelet aggregation with nsPEF, with
evaluation of one growth factor following activation,
PDGF (Zhang, 2008). Measurements of PDGF
release from washed platelets with nsPEF were
compared to PDGF release using bovine thrombin;
PDGF levels with nsPEF were generally close to the
release measured using bovine thrombin (Zhang,
2008).
While these experiments have been very
promising for the proof of concept platelet activation
using nsPEF, there has been a need for a more
complete characterization and benchmarking of this
novel activation method. This paper presents a study
of growth factor release from human platelets by
looking at several growth factors, and reveals
significant differences between thrombin-mediated
activation and nsPEF-mediated activation of
platelets.
2.1 Platelet Rich Plasma (PRP)
Preparation
For each experiment, one unit of human whole blood
from single donor was purchased from a commercial
vendor (Bioreclamation) and shipped following lab
testing for standard pathogens; the vendor used ACD
as an anticoagulant. Blood was therefore 3 days old
at time of PRP preparation. Haematological
measurements are performed on the day of PRP
preparation, including density of red blood cells
(RBCs), platelets (PLTs) and haemoglobin.
Standard preparation of PRP was performed
using a commercial kit and centrifuge (SmartPReP2
APC+, Harvest Technologies) per manufacturer’s
protocol. Briefly, 60mL of whole blood is placed in
separation device and up to 7mL of PRP is
recovered following centrifugation steps, which
usually take about 15 minutes. Typical enrichment
of PLTs is 3 times the amount of the starting density
in whole blood. PRP is aliquoted (1mL per aliquot)
into 4mm cuvettes (Molecular BioProducts catalog
#212373) or 1.5mL Eppendorf tubes for activation
Ex-vivo Platelet Activation using Electric Pulse Stimulation
203

studies and allowed to sit at room temperature until
used for experiment.
2.2 Activation of Platelets
2.2.1 Thrombin-mediated Activation
Reagents were prepared and stored on ice on the day
of experiment. Bovine thrombin (BioPharm
Laboratories catalog #91-010) was prepared in
saline solution (0.9% NaCl) at a stock concentration
to allow for 1:10 (vol/vol) standard dilution in all
experiments.
Bovine thrombin preparation details are below:
124 mg/bottle = 10000 U/bottle in 1 mL 0.9% NaCl
for injection = 10 U/uL. Do 1:10 dilution so that
concentration = 1U/uL. For 50 U experiments, add
50 uL (we used 1, 5, 50, 500, 1000 U for our
experiments of platelet activation with bovine
thrombin). Unless stated – data presented in this
paper will focus on results obtained with 1 U. CaCl2
(Sigma Aldrich) was prepared at stock concentration
to allow for 1:100 (vol/vol) standard dilution in all
experiments but is maintained in these studies at
10mM CaCl2.
We add bovine thrombin to 1 mL of PRP in 4mm
cuvette (Molecular BioProducts catalog #212373)
and allow sample to sit at room temperature;
typically clotting with bovine thrombin occurs
within roughly 30 seconds. The PRP is then
centrifuged at 10,000 rpm for 10 minutes in an
Eppendorf tube. The supernatant is pipetted from
tube and either used in assay immediately or stored
at ≤ -20 C.
2.2.2 Activation Studies: nsPEF-mediated
Activation
For each experiment, we applied electric field pulses
to 1 mL of freshly prepared PRP in 4mm cuvette
(Molecular BioProducts catalog #212373) and allow
sample to sit at room temperature for up to 30
minutes (clotting takes place within roughly 5
minutes). The PRP is then centrifuged at 10,000 rpm
for 10 minutes in a Eppendorf tube. The supernatant
is pipetted from tube and either used in assay
immediately or at ≤ -20 C.
2.2.3 Growth Factor Measurements
All measurements are performed using commercial
enzyme-linked immunosorbent assays (ELISAs)
using manufacturer’s protocols: PDGF (R&D
Systems, #DAA00B), IGF-1 (R&D Systems,
#DG100), EGF (R&D Systems, DEG00), VEGF
(R&D Systems, DVE00).
3 PULSE GENERATOR FOR
PLATELET ACTIVATION AND
EXPERIMENTAL RESULTS
Electric pulse generation relies on a few approaches,
the most common being capacitive energy discharge
and pulse forming networks. Capacitive energy
discharge methods are the simplest, but they provide
pulses that can be very difficult to regulate in
amplitude and duration. Pulse forming networks
organized as lines or an ensemble of passive
elements such as inductors and capacitors are by far
the most common topology to generate short square
pulse with specified pulse width.
The pulse generator for ex-vivo activation of
platelets has been designed and built at Old
Dominion University (ODU) and delivers 300 ns
pulses to the load; the load is a 4-mm cuvette
containing platelet rich plasma. The output voltage
of the instrument is 12 kV and creates an electric
field of 30 kV/cm in the 4 mm cuvettes. The
instrument was designed so that the impedance of
the 4 mm cuvette with platelet rich plasma matches
its output impedance. Typically, the 4 mm cuvette
with platelet rich plasma will behave like a resistive
load, with an impedance of roughly 15 ohm.
The nanosecond pulse generator is powered by
standard 110 V AC and can deliver 1 – 9
nanosecond electric field pulses in a single
sequence. The device also provides a cuvette holder
that is designed for standard electroporation cuvettes
that are commercially available for in vitro
workflows.
The nanosecond pulse generator uses a pulse
forming network to generate the 300 ns pulses. The
pulse forming network consists of a combination of
capacitors and inductors arranged in a Blumlein-line
configuration, as shown in Figure 1. The generator
uses an AC-DC rectifier and a DC-DC converter to
step up the voltage from 110 V AC to about 12 kV
DC. A spark gap switch is used to determine the
output voltage of the pulse forming network.
The cuvette holder is easily accessed through an
opening that is placed on the top of the device.
A picture of the internal components of the
nanosecond pulse electric field generator is shown in
Figure 1.
Generally, experiments for platelet activation
using the instrument described here used electric
BIODEVICES 2013 - International Conference on Biomedical Electronics and Devices
204

Figure 1: Picture of internal architecture of the nanosecond
pulse electric field generator; the pulse forming network is
highlighted in blue.
fields on the order or 30 kV/cm. Five pulses at 5 Hz,
were used for platelet activation here. Previous
experiments at Old Dominion University have
identified the optimal number of pulses for
activation as five.
Figure 2 shows an example of activated platelets
using nanosecond pulse electric fields. The gel like
consistency is easily observed – as a result of
platelet aggregation during activation. There are red
blood cells in the platelet rich plasma typically
separated by the Harvest Technologies instrument –
therefore the platelet gel is red. Other platelet
separation technologies can leave out the red blood
cells – the platelet gel will be yellowish in color.
Figure 2: Example of platelet gel created at GE Global
Research using nanosecond pulse electric fields.
Finally, Figure 3 gives a simple visual
representation of PRP activation in cuvettes: nsPEF
versus negative control (no pulsing). Platelet
aggregation and clot formation prevent the PRP to
flow to the bottom of the cuvette after nanosecond
pulsing; in the control cuvette PRP flows to the
bottom, as no activation or clotting occurs.
Several growth factors were evaluated in terms
of release, using nanosecond pulse electric fields
(nsPEF) and bovine thrombin: TGF – β1, PDGF –
Figure 3: Effects of nsPEF on platelet rich plasma: left
hand side cuvette was used as control (no nsPEF), while
the right hand side cuvette was pulsed with nsPEF. Pulsed
PRP cuvette shows clotting (platelet activation) – not PRP
flow. In the control cuvette the PRP flows to the bottom,
as no clotting occurs. Here cuvettes are turned upside
down.
aa, IGF, VEGF and EGF. The platelet enrichment
obtained was around three times higher compared to
whole blood. Generally platelet rich plasma has a
platelet concentration about three to five times
higher compared to whole blood (Whitlow, 2008).
Platelet-derived growth factor (PDGF) is responsible
with cell replication, stimulates angiogenesis, and
regulates collagen synthesis (Tate and Crane, 1999).
Transforming growth factor-beta (TGF- β)
stimulates undifferentiated mesenchymal cell
proliferation, stimulates angiogenesis and regulates
mitogenic effects of other growth factors (Tate and
Crane, 1999). Vascular endothelial growth factor
(VEGF) stimulates angiogenesis and acts as
mitogenetic factor for endothelial cells (Tate and
Crane, 1999). Epidermal growth factor (EGF)
stimulates angiogenesis and promotes growth and
differentiation of chondrocytes and osteoblasts (Tate
and Crane, 1999). Insulin-like growth factor 1 (IGF-
1) has effects on differentiation, peripheral growth,
and survival in various cells and tissues.
Blood from several human donors was used for
these experiments. As pointed in literature, there are
donor-to-donor variations with respect to amount of
growth factor released upon platelet activation. As a
general trend for the work presented here, we
observed that differences between nsPEF and bovine
thrombin for VEGF and EGF levels can be
considerable.
As expected, nsPEF and bovine thrombin do not
increase IGF-1 levels upon platelet activation
(Everts, 2006). IGF-1 levels for nsPEF and bovine
thrombin are roughly equivalent (data not shown).
The fact that IGF-1 levels in activated platelets are at
the same levels as in non-activated platelets is
Ex-vivo Platelet Activation using Electric Pulse Stimulation
205
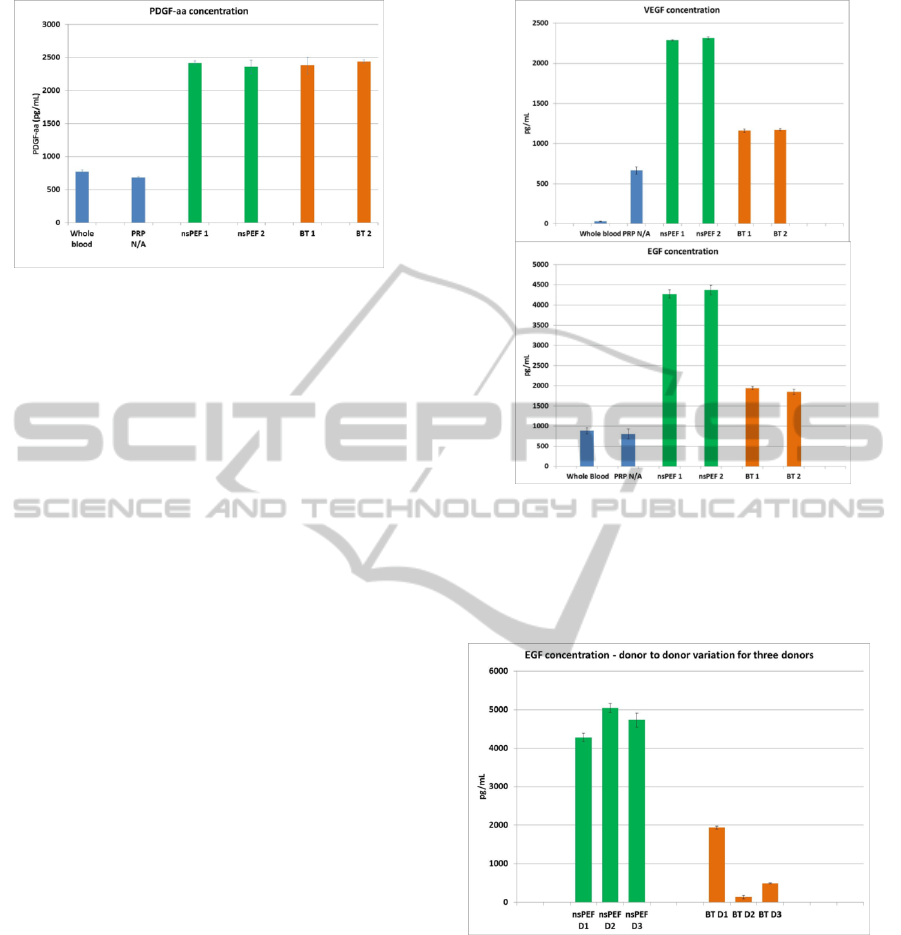
Figure 4: Example of data for PDGF-aa release for nsPEF
compared to bovine thrombin (BT) for one donor; 1 and 2
designate the number of cuvettes tested; from each
cuvette, three samples of supernatant were tested for
growth factor release, and data averaged for each bar
graph.
explained by considering that the plasma pool of
IGF-1 is greater than the platelet pool; IGF-1 is
mainly excreted by the liver in the plasma.
Additionally, PDGF-aa and TGF – β1 levels have
been observed as largely equivalent between nsPEF
and bovine thrombin (TGF – β1 data not shown
here).
Representative data from our studies are shown
here. “nsPEF” indicates the samples activated with
nanosecond pulse electric fields, while “BT”
indicates samples activated with bovine thrombin.
First, PDGF-aa levels nsPEF versus bovine thrombin
are displayed here for a single donor (Figure 4).
We observed that growth factor levels are
significantly increased compared to whole blood and
platelet rich plasma (PRP) that was not activated.
Unless specified, the bovine thrombin activation was
performed with 1 U/ul. Each bar graph was obtained
from a different cuvette – using PRP from the same
donor.
Figure 5 shows that the use of nsPEF increases
the levels of VEGF and EGF compared to negative
controls, whole blood and non-activated platelet rich
plasma. As pointed here, significantly higher levels
of VEGF and EGF seem to be released with nsPEF
compared to bovine thrombin. This was an
unexpected result. Various measurements of growth
factor release with several bovine thrombin
concentrations – from 1 to 1000 units – did not
trigger similar VEGF and EGF levels as obtained
with nsPEF (data not shown here). Therefore the
results in Figures 5 do not seem to be caused by an
insufficient amount of added bovine thrombin.
Finally, if one looks at donor to donor variability
for EGF and VEGF release – our data seem to
indicate much higher variability for bovine thrombin
Figure 5: Example of data for VEGF and EGF release for
nsPEF compared to bovine thrombin (BT) for one donor. 1
and 2 designate the number of cuvettes tested; from each
cuvette, three samples of supernatant were tested for
growth factor release, and data averaged for each bar
graph.
Figure 6: Example of data for EGF release for nsPEF
compared to bovine thrombin (BT), for three donors – D1,
D2 and D3.
compared to nsPEF. As an example, Figure 6
displays EGF release data for three donors, bovine
thrombin (1 U) versus nsPEF.
What is striking about the data in Figure 6 is not
necessarily the high donor to donor variability for
EGF release with bovine thrombin – this has been
noted previously in literature. The much lower
variability of EGF release with nsPEF from donor to
donor is intriguing and unexpected.
BIODEVICES 2013 - International Conference on Biomedical Electronics and Devices
206

4 DISCUSSION OF RESULTS
Experimental data for growth factor release seem to
indicate that using electric pulse stimulation in the
nanosecond range for platelet activation would result
in a different growth factor profile compared to
bovine thrombin. For example, existing data in
literature show that typical physiological platelet
activators – thrombin, ADP, collagen – tend to have
relatively similar growth release profiles for VEGF
(Maloney, 1998). While the use of nsPEF can offer
the advantage of platelet activation without the use
animal based activators already on the market –
bovine thrombin, bovine derived collagen – one
would have to establish the in vivo wound healing
effects of this different growth factor mix.
There are additional questions to be answered
with in vitro experiments before considering a
clinical path, such as the effects of nsPEF on other
components in the platelet rich plasma (white blood
cells - WBC, red blood cells - RBC). Additionally,
one would need to understand how nsPEF act on
platelet rich plasma compositions produced by the
numerous platelet separation machines
commercially available.
Different devices produce various versions of
platelet rich plasmas – RBC count, WBC count,
platelet enrichment, viability of PRP components
can vary. These versions of PRP may not only be
different from a biology point of view, but also they
could exhibit different electrical behaviours, which
may need to be accounted when one would design a
commercial instrument. Finally, experiments
described in this work use typical electroporation
cuvettes that may need additional qualification for
any human in vivo work.
5 CONCLUSIONS
The use of nanosecond electric field pulses for ex-
vivo platelet activation is an exciting novel
technology, which opens promising opportunities for
a truly autologous solution in the platelet gel space,
by accomplishing platelet aggregation and growth
factor release without using animal derived
activators. It should be noted that previous
researchers demonstrated various means for platelet
activation that do not include the use of thrombin or
other bio-chemical vectors – based on the use of
physical means such as ultrasound (Poliachik, 2001),
light (Verhaar, 2008) and high speed centrifugation
(Mazzucco, 2009). However the use of nsPEF for
platelet activation has significant advantages over
previous attempts to bypass the use of biochemical
activators: speed (~ 1 s exposure to electric field
pulses), process control, low cost, simplified
workflow. The wide potential applicability of
platelet gel therapy – healing of non-healing wounds
such as diabetic foot ulcers, haemostasis, and
reduction of wound infection – may be further
fostered by the introduction of this rapid, low cost,
easy access, truly autologous, non-animal derived
platelet activation method.
ACKNOWLEDGEMENTS
The authors of this paper would like to thank
Barbara Hargrave, Richard Heller (Old Dominion
University) and Reginald Smith (GE Global
Research) for valuable discussions and suggestions
throughout this research. The authors thank Yeong-
Jer Chen (Old Dominion University) for building
and providing technical assistance with the
nanosecond pulse generator used for experiments
presented here.
REFERENCES
Tate, K. S. and Crane, D. M., 2010. Platelet rich plasma
grafts in musculoskeletal medicine. In Journal of
Prolotherapy, Volume 2, Issue 2.
Driver, V. R., Hanft, J., Fylling, C. P., Beriou, J. M., 2006.
A prospective, randomized, controlled trial of
autologous platelet-rich plasma gel for the treatment of
diabetic foot ulcers. In Ostomy/Wound Management,
52(6).
Lacci, K. M. and Dardik, A., 2010. Platelet-rich plasma :
support for its use in wound healing. In Yale Journal
of biology and medicine, 83.
Gunaydin, S., McCusker, K., Sari, T., Onur, M., Gurpinar,
A., Sevim, H., Atasoy, P., Yorgancioglu, C., Zorlutuna
Y., 2008. Clinical impact and biomaterial evaluation
of autologous platelet gel in cardiac surgery. In
Perfusion, 23, 179-186.
Alexander, W., 2009. Meeting highlights of the 30th
American College of Clinical Pharmacy annual
meeting. In Pharmacy & Therapeutics, vol. 34, no. 12.
Cada, D. J., Levien, T. Baker, D. E., 2008. Thrombin,
Topical (Recombinant). In Hospital Pharmacy
Volume 43, Number 7, Wolters Kluwer Health, Inc.
Beebe, S. J. and Schoenbach, K. H., 2005. Nanosecond-
pulsed electric fields: a new stimulus to activate
intracellular signalling. In Journal of Biomedicine and
Biotechnology. 4:297-300.
Zhang, J., Blackmore, P. F., Hargrave, B. Y., Xiao, S.,
Beebe, S.J, Schoenbach, K.H., 2008. Nanosecond
Ex-vivo Platelet Activation using Electric Pulse Stimulation
207

electric field pulse (nanopulse): a novel non-ligand
agonist for platelet activation. In Archives of
biochemistry and biophysics, 417.
Whitlow, J., Shackelford, A. G., Sievert, A. N., Sistino, J.
J., 2008. Barriers to the acceptance and use of
autologous platelet gel. In Perfusion, 23: 283–289
Everts, P. A. M., Mahoney, C.B., Hoffmann, J.J.M.L.,
Schonberger, J. P. A. M., Box, H. A. M., van Zundert,
A., Knape, J. T. A., 2006. Platelet rich plasma
preparation using three devices: Implications on
platelet activation and platelet growth factor release. In
Growth Factors. 24:165-171.
Maloney, J. P., Silliman, C.C., Ambruso, D. R., Wang, J.,
Tuder, R. M., Voelkel, N.F., 1998. In vitro release of
vascular endothelial growth factor during platelet
aggregation. In American Journal of Physiology-
Heart and Circulatory Physiology 275:H1054-H1061.
Poliachik, S. L., Chandler, W. L., Mourad, P. D., Ollos, R.
J., and Crum, L. A., 2001. Activation, aggregation and
adhesion of platelets exposed to high-intensity focused
ultrasound. In Ultrasound in Medicine and Biology,
Vol. 27, No. 11, pp. 1567–1576.
Verhaar, R., Dekkers, D. W. C., De Cuyper, I. M.,
Ginsberg, M. H., Korte, D. Verhoeven, A. J., 2008.
UV-C irradiation disrupts platelet surface disulfide
bonds and activates the platelet integrin _IIb_3. In
Blood, Volume 112, Number 13
Mazzucco, L., Balbo, V., Cattana, E., Guaschino, R., and
Borzini, P., 2009. Not every PRP-gel is born equal -
Evaluation of growth factor availability for tissues
through four PRP-gel preparations: Fibrinet®,
RegenPRP-Kit®, Plateltex® and one manual
procedure. In Vox Sanguinis 97, 110–118
BIODEVICES 2013 - International Conference on Biomedical Electronics and Devices
208
