
Cellular Factories
Emerging Technologies for Fabrication of Nanomedicines?
V. Ramos
1
, X. Turon
2
and S. Borros
1
1
Grup d’Enginyeria de Materials, Institut Quimic de Sarria, Universitat Ramon Llull, Barcelona, Spain
2
Department of Bioengineering, Institut Quimic de Sarria, Universitat Ramon Llull
Via Augusta 390, 080017 Barcelona, Spain
Keywords: Bioprocessing, Nanomedicine, Drug Delivery, Metabolomics, Recombinant Dna, Microbial Factories,
Protein Nanocages.
Abstract: The development of innovative nanomedicines requires the implementation of new biocompatible materials
and their efficient assembly into defined nanostructures. Complex and costly synthesis of these materials
can be coped with biological fabrication using microorganism factories, recombinant DNA and metabolic
engineering. Modern bioprocess technologies may have the key for the implementation of tomorrow’s
nanomedicines. This paper specifically focuses on the current state of the art of nanopharmaceuticals and
their future perspectives.
1 INTRODUCTION TO DRUG
DELIVERY
The majority of clinically approved drugs are low
molecular weight molecules (below 10
3
g/mol),
which are often membrane permeable and generally
spread throughout the whole body. As a
consequence drugs reach healthy tissues as well as
disease targets, which may result in unwanted side
effects and/or rapid clearance and elimination. Non-
specific biodistribution also results in a decreased
therapeutic effect due to lowered accumulation at the
target site. An effective approach to decrease side
effects and enhance drug potency makes use of
sophisticated delivery systems, several of which
have crystalized in new approved therapies (Duncan
2003). Over the last years, multidisciplinar
collaboration in biomedical research together with
converging scientific technologies, such as
nanotechnology and biotechnology have led to the
development of modern nanomedicine (Duncan and
Gaspar, 2011).
2 CURRENT STATE OF
NANOMEDICINE
Nanomedicine is an overall term that has been
defined by the European Science Foundation´s
Forward Look Nanomedicine in the following
manner: “Nanomedicine uses nano-sized tools for
the diagnosis, prevention and treatment of disease
and to gain increased understanding of the complex
underlying pathophysiology of disease. The ultimate
goal is improve quality-of-life”.
Modern nanomedicines fit into three groups. The
first group consists of first generation
nanomedicines that have already entered routine
clinical use and they include blockbuster products
and certain products that are of such an age that they
will soon begin to appear as generics. This group is
mainly formed by technologies developed during the
second half of the 20
th
century, such as liposomes
(e.g: liposomal amphotericin B Ambisome (Lopez-
Berestein, 1986) or liposomal doxorubicin Myocet
(Mross et al., 2004)), polymer-protein conjugates
(e.g.: styrene maleic anhydride-neocarcinostatin
Zinostatin Stimaler (Maeda, 2001) or pegylated
adenosine deaminase Adagen (Gaspar et al., 2009))
and polymeric drugs (e.g.: Glu-Ala-Tyr copolymer
Copaxone (Johnson et al. 1995)).
The second group is made of an increasing
209
Ramos V., Turon X. and Borros S..
Cellular Factories - Emerging Technologies for Fabrication of Nanomedicines?.
DOI: 10.5220/0004329202090215
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 209-215
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

number of nanomedicines in clinical development. It
seems certain that a significant number of
nanomedicines based on already approved delivery
systems, such as liposomes and polymer-protein
conjugates, encompassing new bioactives will
continue to reach market approval. In addition, it is
likely that other technologies, such as polymer- or
antibody-drug conjugates (LoRusso et al., 2011),
block co-polymer micelles (Hamaguchi et al., 2005)
and/or nanoparticles (Wohlfart et al., 2011) will
have their first regulatory approval and commercial
success over the next decade, increasing the
confidence of new technology approval.
Finally, the third group comprises innovative
nanotechnologies, mostly still in pre-clinical or even
proof-of-concept stages that may have the potential
to enter clinical development. Many
nanotechnologies are being continuously proposed
for use as nanomedicines, such as carbon nanotubes
(Wu et al., 2009), inorganic nanosized particles
(Goel et al., 2009) or PRINT (particle replication in
non-wetting templates) particles (Canelas et al.,
2009). Significant progress in nanomedicine design
together with the maturing of regulatory aspects
experienced during the last decades are expected to
fertilize the route towards a new paradigm to
diagnosis and therapy.
Although it is difficult to predict the future in
nanomedicine development, the lessons learned
from first generation nanomedicines permits some
speculation about preferred features and avoidable
aspects of tomorrow’s nanomedicines. Hence, it is
important to emphasize that well-defined materials
must be used for future developments, since many
current nanomaterials are inherently heterogeneous.
In addition, nanomedicines should preferably arise
from rational design rather than a let’s try attitude.
For safety reasons, nanomedicines should be
biodegradable to known and non-toxic metabolites
or alternatively be engineered for efficient
elimination via renal and/or hepatobiliary routes in
order to avoid lysosomal storage disorders (Garnett
and Kallinteri, 2006). Another important challenge
is that emerging nanomedicines must be
technologically feasible for large-scale
manufacturing and processing to translate in cost
effective novel therapies. However, fabrication of
nanomedicines via synthetic approaches tends to be
costly and technically difficult due to the large
number of processing and purification steps. The
purpose of this paper is to ponder whether
nanomedicines of the future might be synthesized by
biological means (bioprocessing). Such
biofabrication platforms would represent direct and
cost-effective systems for the production of complex
nanomedicines.
3 FUTURE OF NANOMEDICINE
Evolution has furnished biological processes with an
enviable level of control and specificity, which
translates into exquisitely controlled hierarchical
architectures at the molecular and supramolecular
scale. These elegant structures and precise functions
of biomacromolecules have inspired and continue to
inspire strategies for nanomedicine development.
However, it is likely that the next revolution in
nanomedicine research will be fuelled by
convergence of molecular and cellular biology with
genomics, engineering and physical sciences to
biofabricate nanomedicines (Sharp et al., 2011)
rather than by construction of (bio)inspired
macromolecular synthetic mimics or biological-
synthetic hybrid structures (Pasparakis et al., 2010).
The possibility to use the cellular machinery to
entirely biosynthesize nanomedicines, would open
the way to the development of innovative
nanomedicines from new biocompatible materials
produced by cost-effective fabrication methods, in
contrast to difficult entirely synthetic methods. The
biological fabrication of materials, mostly carried
out by microorganisms, has historically provided
biomacromolecules with wide-spectrum biomedical
applications, including drugs (Engels et al., 2008),
polymers (Liu et al., 2011), proteins (Ferrer-Miralles
et al., 2009) and nucleic acids. Although
microorganisms might be simply seen as reaction
vessels for bioproduction, development of genetic
and metabolic engineering is likely to render
efficient platforms capable of producing complex
nanomedicines, such as polymer-drug conjugates,
protein nanoparticles or other nanoscale entities. The
tremendous therapeutic potential of such organized
and functional materials in nanomedicine prompts
serious consideration of further exploitation of cell
factories and recombinant DNA technologies as
powerful alternatives to chemical synthesis. For this
purpose, heterologous biosynthesis in engineering-
and process-friendly hosts, such as Escherichia coli
or Saccharomyces cerevisiae, of components and
their subsequent assembly into finished functional
nanomedicines, emerges as a promising technically
feasible and cost-effective platform.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
210

4 THE QUESTION IS: CAN
THESE NEW BIOPROCESSED
NANOMEDICINES BE MADE?
4.1 Polymer-Drug Conjugates
Microorganism-produced polymers are known
interesting alternatives to synthetic polymers, as they
are non-toxic, biocompatible and biodegradable
materials. According to their chemical structure,
biopolymers can be distinguished between
polysaccharides, such as hyaluronic acid (Leonelli et
al., 2008), polyamides, such as poly(γ-glutamic acid)
(Choi et al., 2004), and polyesters, such as
polyhydroxyalkanoates (Kim et al., 2009). Most of
these polymers contain amenable sites for chemical
modification – i.e. ligand conjugation or
functionality introduction – that can render
appropriate polymer tailoring for biomedical
applications. Chemical conjugation of drugs to such
polymers has been widely explored to produce
polymer-drug conjugates that have been evaluated as
potential therapies for cancer (Leonelli et al., 2008)
and inflammatory (Yang et al., 2008) diseases. Some
of these conjugates have had or are experiencing
notable success, such as poly(glutamic acid)-
paclitaxel conjugate (Opaxio), which is currently
under phase III clinical evaluation (Galic et al.,
2011). However, the production of such
macromolecular constructs is often characterized by
difficulties in their manufacture and processing. The
reason for such costly development is the
requirement of reproducible and specific procedures
for chemical conjugation of the drug, followed by
efficient purification of unreacted materials and by-
products. Since a great number of drugs are obtained
or can be obtained by microbial production, such as
anti-cancer blockbusters doxorubicin and Colombo,
1999) and paclitaxel (Engels et al., 2008), it is not
difficult to envisage that some of these polymer-drug
conjugates could be obtained directly in bioprocess
factories as a single final product. The development
of a microorganism-based platforms capable of
simultaneous production of both precursors – i.e. the
drug and the polymer – followed by appropriate
biotransformation mechanisms for successful
conjugation of these precursors into organized
nanostructures emerges as a promising system for
the production of polymer-drug conjugates in a fast,
technically feasible and cost effective manner
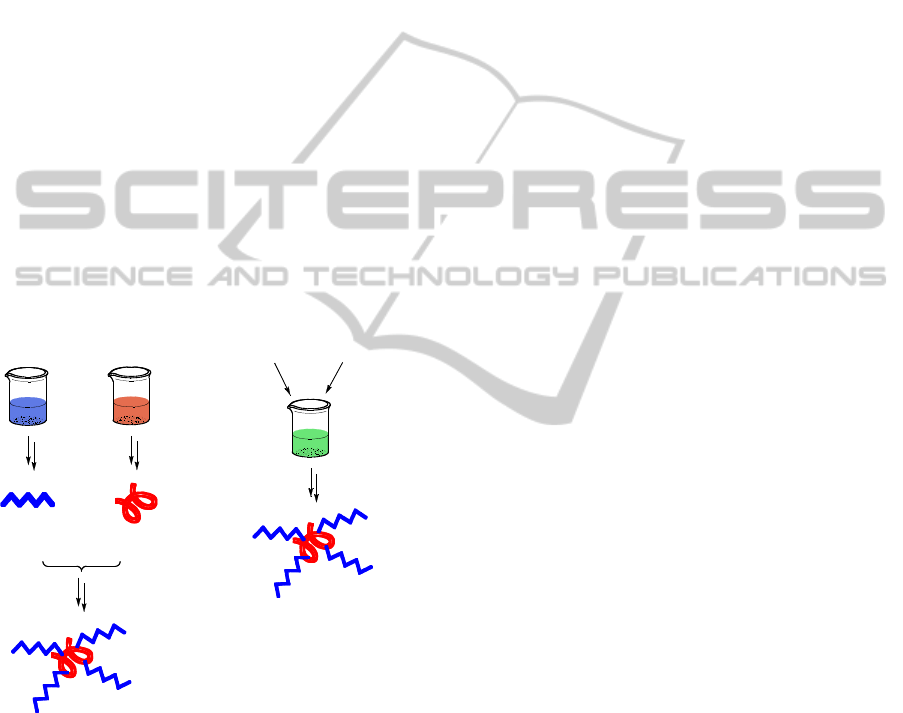
(figure 1).
Notably, the main current limitation is the
introduction of cellular mechanisms capable of
conjugating the drug to the polymer chains inside
modified microorganisms. Such systems would
probably require the introduction of sets of enzymes
capable of chemically linking the drug to the
polymer in a site-specific, robust and reproducible
manner. To the best of our knowledge, there are no
references in the literature about known enzymes
that mediate drug conjugation to polymers.
However, research in this area is likely to identify
enzymes capable of mediating specific polymer-drug
conjugation. This is supported by the fact that a few
enzyme-based approaches for peptide ligation have
been already described. For example, sortase is an
extensively studied transpeptidase found in the cell
envelope of many Gram-positive bacteria that
mediates transpeptidation by recognition of specific
terminal aminoacid motifs at the C- and N-terminal
of its substrates and ligands, respectively (Mao et al.,
2004). Since sortase has shown transpeptidase
activity in other non-amino acid primary amine-
containing substrates, it is likely that the chemical
structure of polymer and drug molecules may be
engineered to make use of such enzyme-based
coupling strategies (Ta et al., 2012). Another
promising family of enzymes to be considered for
enzymatic-based coupling of polymers and drugs are
glycosyltransferases (Boltje et al., 2009); (Wagner
and Pesnot, 2010).
Figure 1: Synthesis of polymer-drug conjugates: synthetic
vs. bioprocess approach.
4.2 Polymer-Protein Conjugates
Deficiency of specific proteins or non-functional
versions of biologically relevant proteins may derive
in diverse pathologies. Such disorders can be
addressed clinically by administration of the missing
protein to reach adequate physiological concentra-
tions. However, in many cases therapeutic proteins
Engineered
microorganisms
Appropriate
feedstock
Polymer
Synthesis
D
r
ug
Synthesis
Chemical
Conjugation
drug
polymer
Polymer-Drug Conjugate
CellularFactories-EmergingTechnologiesforFabricationofNanomedicines?
211

are very difficult to obtain from their natural sources
and therefore bioprocess platforms using
recombinant DNA technologies have been
developed. Potent and relatively cost-effective
production procedures can be achieved by
cultivation of conveniently modified microbial cells,
such as bacteria and yeast (Ferrer-Miralles et al.,
2009).
Although there is an increasing number of
approved recombinant proteins to be used as
biopharmaceuticals, many of these therapeutic
proteins face some limitations, which include short
circulating half-life, immunogenicity, low solubility
and proteolytic degradation (Duncan, 2003). A few
strategies have been developed in order to improve
their pharmacological properties for safer and more
efficient use. Such strategies include changes in their
amino acid sequence to reduce immunogenicity and
proteolytic cleavage, conjugation to other proteins,
such as albumin (Kurtzhals et al., 1995), or
conjugation to natural or synthetic polymers
(Roberts et al., 2012). The most efficient and
versatile strategy so far consists on the chemical
coupling of poly(ethylene glycol) (PEG). PEG
conjugation can protect therapeutic proteins from
premature clearance, proteolytic enzyme degradation
and immunogenicity. In addition, PEGylation
increases the apparent size of proteins, thus reducing
renal filtration, which results in extended circulating
half-life (Veronese and Pasut, 2005). Undoubtedly,
PEGylation has made possible the clinical use of
certain therapeutic proteins, whose administration
compliance would otherwise be unfeasible. Despite
clinical success, PEGylation of biologically active
proteins may present drawbacks with respect to
biopharmaceutical development and production,
since additional in vitro processing and purification
steps are required. Furthermore, the biological
function of the therapeutic protein may be impaired,
if chemical coupling takes place in the vicinity of its
bioactive site. In addition, PEG is not biodegradable
and may cause severe side effects, such as
vacuolation of organs upon chronic administration.
A wide arsenal of both synthetic and natural
polymers, such as poly[N-(2-hydroxypropyl)-
methacrylamide] (Johnson et al., 2012) and
polyvinylpyrrolidone (Shibata et al., 2005) or
polysialic acid (Pisal et al., 2010) or hyaluronic acid
(Ferguson et al., 2010), have been explored as
alternatives to PEG, however these systems do not
avoid the need for additional processing and
purification steps in order to obtain the final
polymer-protein conjugates, and finally they have
not shown superior performance than PEG.
Similarly as discussed earlier for polymer-drug
conjugates, the development of bioprocess platforms
capable of producing polymer-protein conjugates,
either during protein synthesis (co-translational
modification) or on finished proteins (post-
translational modification), emerges as a promising
alternative to polymer modification via chemical-
based coupling strategies (figure 2). In this case,
protein processing may also provide appropriate
targeting signals to traffic the therapeutic protein to
specific target sites.
A few alternative strategies to avoid synthetic
post-modification strategies have been already
proposed during the recent years, including
glycosylation (Flintegaard et al., 2010) and genetic
fusion of carrier proteins and polypetides (Cleland
and Geething 2012). Modification of therapeutic
proteins with glycans to prolong their in vivo half-
life can be achieved by introducing mutations in
their amino acid sequence in order to establish
glycosylation sites. Glycosylation at these sites
occurs via glycosyltransferase enzymes in protein
processing events, either at the rough endoplasmic
reticulum or the golgi apparatus. For success, the
host platform requires to be suitably
glycoengineered in order to correctly biosynthesize
the therapeutic glycoprotein. Following this strategy,
a first successful pharmaceutical product, Aranesp
(glycoengineered erythropoietin), received market
approval in 2001 and it is expected that others will
follow. Although specific glycosylation might be
useful for prolonging half-life, it may result in
unwanted retargeting or increased immunogenicity.
An emerging alternative to PEGylation and
glycosylation of proteins is the post-translational
enzymatic-conjugation of natural polysaccharides
found in the human body, such as polysialic acid or
hyaluronic acid. The hypothesis behind this strategy
is that glycoengineered microorganisms could be
used to produce PSA- or HyA-conjugated proteins in
a single fermentation without the need for in vitro
chemical modification.
Genetic fusion of either natural proteins, such as
albumin (Sheffield et al., 2004), or unstructured
polypeptide sequences of hydrophilic amino acids to
either C,N-terminus or both termini of a
recombinant protein provides a simple way to
prolong plasma half-life and to diminish
immunogenicity and proteolytic cleavage of
biopharmaceuticals. Genetic fusion strategy allows
biotechnological production of polymer-conjugated
therapeutic proteins as one single product without
the need of additional processing and purification
steps. In addition, this system can be easily adjusted
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
212
to match the pharmacological needs by varying the
polypeptide length and composition. Alternatively,
targeting signals can be generated at C- or N-
terminus to enable protein trafficking towards target
tissues or cells. An interesting advantage of this
technology is that in contrast to PEGylation genetic
fusion of polypeptides renders a homogenous,
monodisperse product with a defined chemical
composition.
PASylation and XTEN technologies are two
proprietary genetic fusion technologies that consist
of disordered polypeptide sequences of Pro, Ala and
Ser, and unstructured polypeptide containing Ala,
Glu, Gly, Pro, Ser and Thr, respectively. It has been
claimed that these technologies may reduce the cost
of goods by up to 10-fold relative to PEGylation
technologies.
A parallel alternative to genetic fusion is the
polyglutamation and polyglycilation of therapeutic
proteins, which consists of post-translational
enzymatic-conjugation at the C-terminus of
polymeric Glu and Gly, respectively (Janke et al.,
2008).
Figure 2: Synthesis of polymer-protein conjugates:
synthetic vs. bioprocess approach.
4.3 Protein Cages and Nanoparticle
Drug Encapsulation
Drug delivery systems based on drug encapsulation
have been largely explored as potential therapeutic
agents. In general, drug encapsulation enhances drug
efficacy and reduces unwanted effects of free drug
during trafficking to the target site. Lipid (mainly
liposomes) and polymeric nanoparticles (i.e. PLA
(Krause et al., 1985) or Abraxane (Zhao and Astruc,
2012)) have been under continuous development
during the last decades and some products have
already received market approval. These
nanomedicines present some advantages, when
compared to polymer-drug conjugation, including
the protection of premature drug degradation and
restricted interaction with the biological
environment, preferential absorption into a selected
tissue due to their nanoparticulate nature,
bioavailability and retention time. Molecular
organization, shape and size dispersion and drug
encapsulation efficiency of these constructs is
achieved by mechanical and chemical approaches.
However, these constructs are obtained as rather
heterogeneous mixtures. In addition, most of these
particles require surface functionalization to enhance
their pharmacological properties, mainly their half-
life, and to present targeting motifs for specific and
efficient trafficking to diseased tissues or cells.
Inspired by the monodisperse nature of viral
particles and intracellular nanocompartments, it has
been hypothesized that if properly adapted, these
nanostructures could be turned into potent
nanomedicine platforms. Additionally, due to
evolution, viral particles posses specific targeting
and cell-entry machinery, which are highly sought
features in nanomedicine systems. Adaptation of
viral particles as nanomedicine constructs via
conventional chemical techniques would require
casting of the genetic material and maintenance of
the structural capsid for subsequent drug loading or
conjugation and modification. However, one of the
main limitations for using these constructs is the
difficulty to have access to sufficient material of
empty viral capsids, since viruses are obtained by
culturing of host cells. Even if large amounts of viral
capsids were available, chemical conjugation
processes would be complex and expensive and
would probably result in random attachment patterns
and undesirable heterogeneity. For these reasons, a
versatile bioprocess platform for the production of
virus-like capsids or any other supramolecular
structure suitable for accommodating drugs, small
proteins or even nucleic acids in a cost-effective
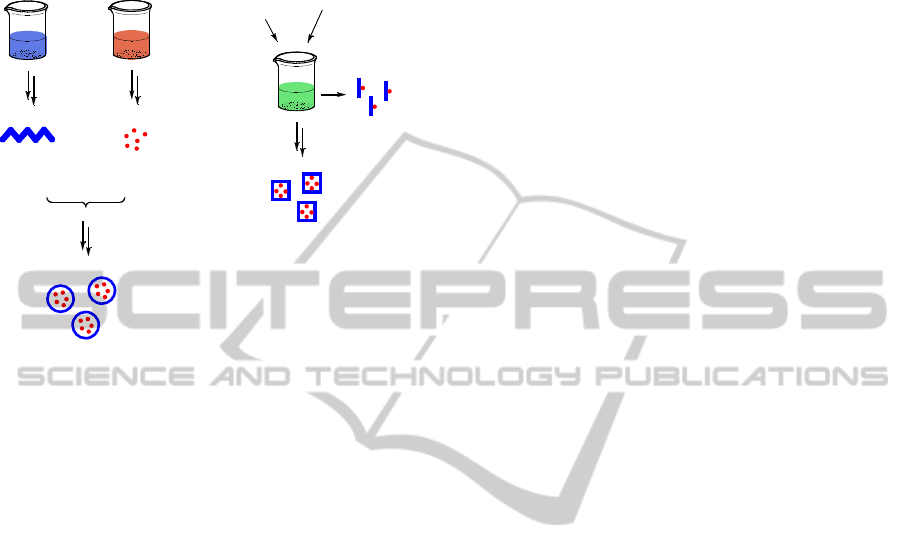
manner would be highly appealing (figure 3).
In this scenario, the development of bioprocess
platforms capable of producing capsid proteins
followed by macromolecular self-assembly could be
exploited to engineer materials for encapsulation of
active principles. Protein-based capsids are
interesting vehicles for delivery applications, since
they are biocompatible and their versatility of design
would allow protein engineering to enhance vital
Engineered
microorganisms
Appropriate
feedstock
Polymer
Synthesis
Protein
Synthesis
Chemical
Conjugation
protein
polymer
Polymer-Drug Conjugate
CellularFactories-EmergingTechnologiesforFabricationofNanomedicines?
213
pharmacokinetic properties, such as prolonged half-
life, enhanced proteolytic resistance and reduced
immunogenicity.
Figure 3: Synthesis of drug-nanoparticles: synthetic vs.
bioprocess approach.
Mechanisms directing drug encapsulation within
capsids, similar to the ones discussed for enzymatic
polymer-drug or polymer-protein conjugation should
be designed to direct drug conjugation to the inner
surface of the capsid. A few early proof of concept
works have demonstrated that it is feasible to
encapsulate small enzymes in the interior of protein-
based bacterial organelles both by specific
enzymatic-based conjugation strategies at the inner
side of the capsid proteins and by gene fusion of
capsid and enzyme proteins (Fan et al., 2010). Upon
macromolecular self-assembly, successful enzyme
encapsulation inside the capsid was observed.
It is likely that the development of enzymatic-
based conjugation strategies and gene fusion
techniques to create specific docking sites in the
interior of protein nanocages will not only allow the
encapsulation of a wide range of therapeutic
molecules, such as small drugs, therapeutic proteins,
nucleic acids and imaging agents, but also the
introduction of cell- or tissue-specific targeting
motifs on the exterior and particle disassembly
mechanisms for efficient release of the therapeutic
load at the target site.
5 CONCLUDING REMARKS
Innovative nanoengineering together with increased
knowledge arising from genomics, proteomics and
metabolomics research brings exciting novel
opportunities for nanomedicine development. There
is a real chance to spur on modern nanomedicine
development, as too many new nanomedicines still
use old strategies and old drugs as the bioactive.
Bioprocessing in the broadest conception of this
term, including fermentation, biotransformation and
downstream separation techniques in favour of new
nanomedicine engineering may open the future to
obtain more specific, defined and potent
nanomedicine systems to improve patient therapy.
REFERENCES
Boltje, T. J., Buskas, T., Boons, G.-J., 2009. Opportunities
and challenges in synthetic oligosaccharide and
glycoconjugate research. Nat Chem, 1(8), pp.611-622.
Canelas, D. A., Herlihy, K. P., DeSimone, J. M., 2009.
Top-down particle fabrication: control of size and
shape for diagnostic imaging and drug delivery. Wiley
Interdisciplinary Reviews: Nanomedicine and
Nanobiotechnology, 1(4), pp.391-404.
Choi, S.-hyun et al., 2004. Production of Microbial
Biopolymer , Poly ( γ-glutamic acid ) by Bacillus
subtilis BS 62. Agricultural Chemistry &
Biotechnology, 47(2), pp.60-64.
Cleland, J., Geething, N., 2012. A Novel Long-Acting
Human Growth Hormone Fusion Protein ( VRS-317 ):
Enhanced In Vivo Potency and Half-Life. Journal of
Pharmaceutical Sciences, 101(8), pp.2744-2754.
Duncan, R., 2003. The dawning era of polymer
therapeutics. Nature reviews. Drug discovery, 2(5),
pp.347-60.
Duncan, R., Gaspar, R., 2011. Nanomedicine(s) under the
Microscope. Molecular Pharmaceutics, 8, pp.2101 -
2141.
Engels, B., Dahm, P., Jennewein, S., 2008. Metabolic
engineering of taxadiene biosynthesis in yeast as a first
step towards Taxol (Paclitaxel) production. Metabolic
engineering, 10(3-4), pp.201-6.
Fan, C. et al., 2010. Short N-terminal sequences package
proteins into bacterial microcompartments.
Proceedings of the National Academy of Sciences of
the United States of America, 107(16), pp.7509-14.
Ferguson, E. L., Alshame, A.M.J., Thomas, D. W., 2010.
Evaluation of hyaluronic acid–protein conjugates for
polymer masked–unmasked protein therapy.
International Journal of Pharmaceutics, 402(1–2),
pp.95-102.
Ferrer-Miralles, N. et al., 2009. Microbial factories for
recombinant pharmaceuticals. Microbial cell factories,
8, p.17.
Flintegaard, T. V. et al., 2010. N-glycosylation increases
the circulatory half-life of human growth hormone.
Endocrinology, 151(11), pp.5326-36.
Galic, V. L. et al., 2011. Paclitaxel poliglumex for ovarian
Engineered
microorganisms
Appropriate
feedstock
Carrier
Synthesis
Drug
Synthesis
Chemical Conjugation
Drug Encapsulation
Surface Modification
...
carrier system
Drug-Nanoparticle
drug
drug-macromolecule
monomer
Drug-Nanoparticle
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
214

cancer. Expert Opinion on Investigational Drugs,
20(6), pp.813-821.
Garnett, M. C., Kallinteri, P., 2006. Nanomedicines and
nanotoxicology: some physiological principles.
Occupational medicine, 56(5), pp.307-11.
Gaspar, H. B. et al., 2009. How I treat ADA deficiency.
Blood, 114(17), pp.3524-3532.
Goel, R. et al., 2009. Biodistribution of TNF-α-coated
gold nanoparticles in an in vivo model system.
Nanomedicine, 4(4), pp.401-410.
Hamaguchi, T. et al., 2005. NK105, a paclitaxel-
incorporating micellar nanoparticle formulation, can
extend in vivo antitumour activity and reduce the
neurotoxicity of paclitaxel. Br J Cancer, 92(7),
pp.1240-1246.
Hutchinson, C. R., Colombo, A. L., 1999. Genetic
engineering of doxorubicin production in
Streptomyces peucetius: a review. Journal of
Industrial Microbiology & Biotechnology, 23(1),
pp.647-652.
Janke, C., Rogowski, K., van Dijk, J., 2008.
Polyglutamylation: a fine-regulator of protein
function? EMBO Rep, 9(7), pp.636-641.
Johnson, K. P. et al., 1995. Copolymer 1 reduces relapse
rate and improves disability in relapsingremitting
multiple sclerosis: Results of a phase III multicenter,
doubleblind, placebocontrolled trial. Neurology,
45(7), pp.1268-1276.
Johnson, R. N., Kopečková, P., Kopeček, J., 2012.
Biological activity of anti-CD20 multivalent HPMA
copolymer-Fab’ conjugates. Biomacromolecules,
13(3), pp.727-35.
Kim, H.-N. et al., 2009. Enzymatic synthesis of a drug
delivery system based on polyhydroxyalkanoate-
protein block copolymers. Chemical communications,
7345(46), pp.7104-6.
Krause, H.-J., Schwarz, A., Rohdewald, P., 1985.
Polylactic acid nanoparticles, a colloidal drug delivery
system for lipophilic drugs. International Journal of
Pharmaceutics, 27(2–3), pp.145-155.
Kurtzhals, P. et al., 1995. Albumin binding of insulins
acylated. , 731, pp.725-731.
Leonelli, F. et al., 2008. Design, synthesis and applications
of hyaluronic acid-paclitaxel bioconjugates.
Molecules, 13(2), pp.360-78.
Liu, L. et al., 2011. Microbial production of hyaluronic
acid: current state, challenges, and perspectives.
Microbial cell factories, 10, p.99.
LoRusso, P. M. et al., 2011. Trastuzumab Emtansine: A
Unique Antibody-Drug Conjugate in Development for
Human Epidermal Growth Factor Receptor 2–Positive
Cancer. Clinical Cancer Research, 17(20), pp.6437-
6447.
Lopez-Berestein, G., 1986. Liposomal Amphotericin B in
the Treatment of Fungal Infections. Annals of Internal
Medicine, 105(1), pp.130-131.
Maeda, H., 2001. SMANCS and polymer-conjugated
macromolecular drugs: advantages in cancer
chemotherapy. Advanced Drug Delivery Reviews,
46(1–3), pp.169-185.
Mao, H. et al., 2004. Sortase-mediated protein ligation: a
new method for protein engineering. Journal of the
American Chemical Society, 126(9), pp.2670-1.
Mross, K. et al., 2004. Pharmacokinetics of liposomal
doxorubicin (TLC-D99; Myocet) in patients with solid
tumors: an open-label, single-dose study. Cancer
Chemotherapy and Pharmacology, 54(6), pp.514-524.
Pasparakis, G. et al., 2010. Controlled polymer synthesis--
from biomimicry towards synthetic biology. Chemical
Society reviews, 39(1), pp.286-300.
Pisal, D. S., Kosloski, M. P., Balu-Iyer, S. V., 2010.
Delivery of therapeutic proteins. Journal of
Pharmaceutical Sciences, 99(6), pp.2557-2575.
Roberts, M. J., Bentley, M. D., Harris, J. M., 2012.
Chemistry for peptide and protein PEGylation.
Advanced drug delivery reviews, 54, pp.459-476.
Sharp, P. A. et al., 2011. The Third Revolution: The
Convergence of the Life Sciences , Physical Sciences
and Engineering.
Sheffield, W. P. et al., 2004. Effects of genetic fusion of
factor IX to albumin on in vivo clearance in mice and
rabbits. British Journal of Haematology, 126(4),
pp.565-573.
Shibata, H., Nakagawa, S., Tsutsumi, Y., 2005.
Optimization of protein therapies by polymer-
conjugation as an effective DDS. Molecules, 10(1),
pp.162-80.
Ta, H. T., Peter, K., Hagemeyer, C. E., 2012. Enzymatic
antibody tagging: toward a universal biocompatible
targeting tool. Trends in cardiovascular medicine,
22(4), pp.105-11.
Veronese, F., Pasut, G., 2005. PEGylation, successful
approach to drug delivery. Drug discovery today,
10(21), pp.1451-8.
Wagner, G. K., Pesnot, T., 2010. Glycosyltransferases and
their Assays. ChemBioChem, 11(14), pp.1939-1949.
Wohlfart, S. et al., 2011. Efficient Chemotherapy of Rat
Glioblastoma Using Doxorubicin-Loaded PLGA
Nanoparticles with Different Stabilizers. PLoS ONE,
6(5), p.e19121.
Wu, W. et al., 2009. Covalently combining carbon
nanotubes with anticancer agent: preparation and
antitumor activity. ACS nano, 3(9), pp.2740-50.
Yang, S. C. et al., 2008. Polyketal copolymers: a new
acid-sensitive delivery vehicle for treating acute
inflammatory diseases. Bioconjugate chemistry, 19(6),
pp.1164-9.
Zhao, P., Astruc, D., 2012. Docetaxel Nanotechnology in
Anticancer Therapy. ChemMedChem, 7(6), pp.952-
972.
CellularFactories-EmergingTechnologiesforFabricationofNanomedicines?
215
