
Solid-state Ag
+
Ion Migration for the Controlled Precipitation of PbS
Quantum Dots in Glasses
Kai Xu and Jong Heo
Department of Materials Science and Engineering, and Division of Advanced Nuclear Engineering, Pohang,
University of Science and Technology (POSTECH), Nam-Gu, Pohang, South Korea
Keywords: PbS Quantum Dots, Ag
+
Ion Migration, Heat Treatment, Glasses.
Abstract: Precipitation of PbS quantum dots (QDs) controlled by solid-state Ag
+
ion migration and subsequent
thermal treatment was investigated. Ag
+
ions migrated from Ag paste applied on the surface into glass at
320°C. After following heat treatment, PbS QDs formed in the near surface area where Ag paste was
coated. Sizes of the PbS QDs were larger in Ag
+
-migrated surface regions than those in Ag
+
-free glasses,
and PbS QDs can grow at temperatures as low as 420 and 430°C. Ag nanoparticles (NPs) also formed
during the thermal treatment. These results suggest that Ag NPs supplied the nucleating sites and promote
the formation of PbS QDs in glasses. The spatial distribution of PbS QDs in glasses can also be controlled
through solid-state Ag
+
ion migration.
1 INTRODUCTION
Lead sulfide (PbS) has a narrow-gap energy (E
g
=
0.41 eV at 298 K) and a large exciton Bohr radius
(a
B
= 18 nm), which allows PbS quantum dots (QDs)
to have size-tunable optical properties in near-
infrared spectra (Wise, 2000). Glasses are suitable
matrices to host semiconductor QDs, because they
can prevent the aggregation of QDs and have the
high chemical stability (Woggon, 1997). Therefore,
glasses containing PbS QDs have the potential
applications as saturable absorbers for near-infrared
lasers (Malyarevich et al., 2008) and in amplifiers
for fiber-optic telecommunication (Heo and Liu,
2007).
Thermal treatment of the precursor glass is the
most common method of precipitating QDs in
glasses (Borrelli and Smith, 1994). Ion implantation
and femtosecond laser irradiation have been
attempted to control the spatial distribution of QDs
in glasses. For example, ion implantation can induce
the formation of PbS QDs within hundreds of
nanometers from the surface of glasses (Lamaestre
et al., 2005). Femtosecond laser irradiation can also
control the spatial precipitation of PbS QDs inside
glasses (Liu et al., 2010). However, these external
fields always cause the serious damages on the
parent glasses.
Noble metallic nanoparticles (NPs), such as Ag
and Au NPs, are well-known as nucleating agents
for controlled crystallization of glasses (Stookey,
1959) or controlled shape and size of PbS
nanocrystals in liquid solutions (Yong et al., 2006).
Recently, precipitation of Ag NPs as nucleating
agents to control the formation of PbS QDs in
glasses has been reported (Xu et al., 2011). A few
tens of parts per million (ppm) of Ag
+
ions were
added in molten glass batches, and precursor glasses
were prepared by the melt-quenching method. After
heat treatment, intensities of the absorption and
photoluminescence (PL) from PbS QDs increased
with the addition of Ag. This was attributed to the
increased number density of PbS QDs with
increasing Ag. However, the maximum solubility of
Ag
+
ions in molten glasses was only ~40 ppm, and
this limited concentration of Ag
+
ions made the
control of PbS QDs precipitation in glasses difficult.
Ion-exchange method has been extensively used
to fabricate optical waveguides in Na-glasses
(Najafi, 1992). This method can incorporate the
large amount of Ag
+
ions in the glass surface
compared to melt-quenching method. Therefore,
Ag
+
ions were incorporated into glasses by dipping
the glasses into AgNO
3
solution (Xu and Heo,
2012a) or melt (Xu and Heo, 2012b). After thermal
treatment, the size of PbS QDs precipitated in the
Ag
+
ion-exchanged surface regions was larger than
that in Ag
+
-free regions. However, the long ion-
83
Xu K. and Heo J..
Solid-state Ag+ Ion Migration for the Controlled Precipitation of PbS Quantum Dots in Glasses.
DOI: 10.5220/0004335500830087
In Proceedings of the International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS-2013), pages 83-87
ISBN: 978-989-8565-44-0
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
exchange duration in AgNO
3
solution causes the
contamination of the glass surface. The migration of
Ag
+
ions was very fast inside the AgNO
3
melt, and it
makes the control of amount of Ag
+
ions difficult.
The solid-state Ag
+
diffusion is proposed as an
alternative approach to incorporate the Ag
+
ions into
glasses (Najafi, 1992).
This paper reports the solid-state Ag
+
migration
into glass for the controlled formation PbS QDs in
glasses. Ag paste was used as the source of Ag
+
ions
and heat was applied to induce Ag
+
ion diffusion. Ag
NPs and PbS QDs were precipitated after subsequent
thermal treatment. PL spectra show that PbS QDs
can form into larger sizes and preferentially
precipitate in the Ag
+
-migrated regions.
2 EXPERIMENTS
A glass with a nominal composition (mol %) of
50SiO
2
- 35Na
2
O - 5Al
2
O
3
- 8ZnO - 2ZnS - 0.8 PbO
was prepared using melt-quenching. Starting
powders of ~25 g were ground in ethanol using ZrO
2
balls. The mixtures were kept in an oven at 110°C
for 24 h to remove ethanol and moisture, then
melted in an alumina crucible at ~1350°C for 45
min. The melts were poured into a pre-heated brass
mold and pressed to a thickness of ~1.5 mm using an
iron plate. The glass was annealed at 350°C for 3 h
to release the thermal stress, then cut into pieces of
~1.0×1.0 cm. Finally, the pieces were optically
polished to the thickness of ~1.0 mm.
One side of glass was coated with 0.1-mm-thick
Ag paste, then heat-treated at 320°C for 2 h to allow
Ag
+
ion migration. Afterwards, remaining Ag paste
on glass surface was removed using acetone, and
specimens were further heat-treated for 10 h at 420,
430, 440 or 450°C, respectively, to induce
precipitation of Ag NPs and PbS QDs.
Formation of QDs was confirmed using a
transmission electron microscope (TEM) under an
accelerating voltage of 200 kV. Oxidation states of
silver in glasses were identified by X-ray
photoelectron spectroscopy (XPS) using Mg-K (hυ
= 1253.6 eV) radiation. The depth of Ag penetration
was analyzed using energy dispersive X-ray
spectroscopy (EDX) after polishing a cross-section
of the glass. PL spectra were recorded using an 800-
nm excitation beam from a continuous-wave Ti-
sapphire laser. Signals were collected and amplified
using a combination of a mechanical chopper of 50-
Hz frequency, a 1/4 m monochromator, an InGaAs
detector and a lock-in amplifier system. All
measurements were performed at room temperature.
3 RESULTS & DISCUSSION
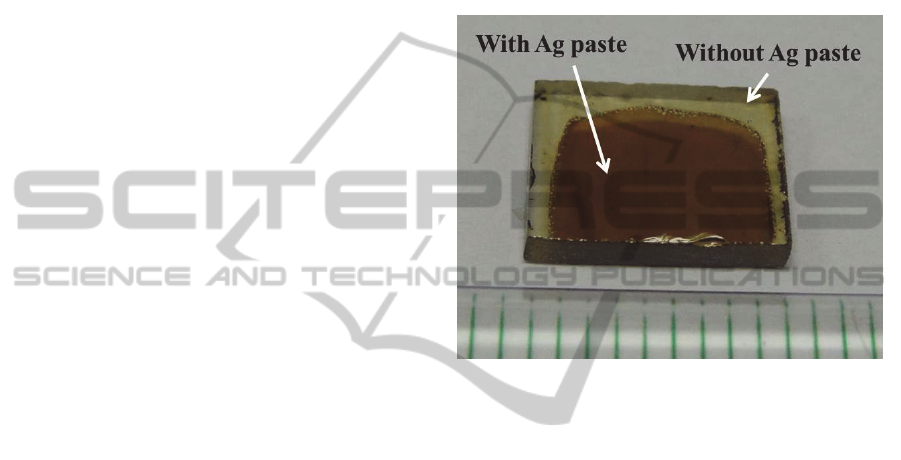
3.1 Appearance of Glasses
After Ag
+
ion migration at 320°C and heat treatment
at temperatures ≤ 430°C, the color of the glass
surface that was coated with Ag paste turned into
dark brown. The region without Ag paste did not
show any color change and remained yellowish
(Figure 1).
Figure 1: Photograph of the glass after Ag
+
ion migration
at 320°C for 2 h and heat treatment at 430°C for 10 h.
3.2 Formation of PbS QDs
To identify the crystal structure precipitated in the
glass, a TEM specimen was prepared from the dark
brown surface using the focused ion beam milling. A
TEM micrograph of a single crystal in Figure 2
shows the fringe spacing of ~0.17 nm. This is
similar to the (222) plane spacing of bulk PbS, and
therefore, we believe PbS QDs formed in the glass
surface that was coated with Ag paste.
Unfortunately, the crystals relative with Ag could
not be identified from the TEM images.
3.3 PL Spectra of PbS QDs in Glasses
PL spectra were recorded to demonstrate the effect
of Ag
+
ion migration on the formation of PbS QDs.
The incident laser excited only the glass surface
where Ag
+
ions migrated. Clear PL bands from PbS
QDs were observed (Figure 3a). The center
wavelengths of PL shifted from ~1160, ~1260,
~1390 to ~1530 nm when heat-treatment
temperatures increased from 420, 430, 440 to 450°C.
PL spectra from glasses without Ag
+
ion migration
were also recorded as shown in Figure 3b (Xu and
PHOTOPTICS2013-InternationalConferenceonPhotonics,OpticsandLaserTechnology
84
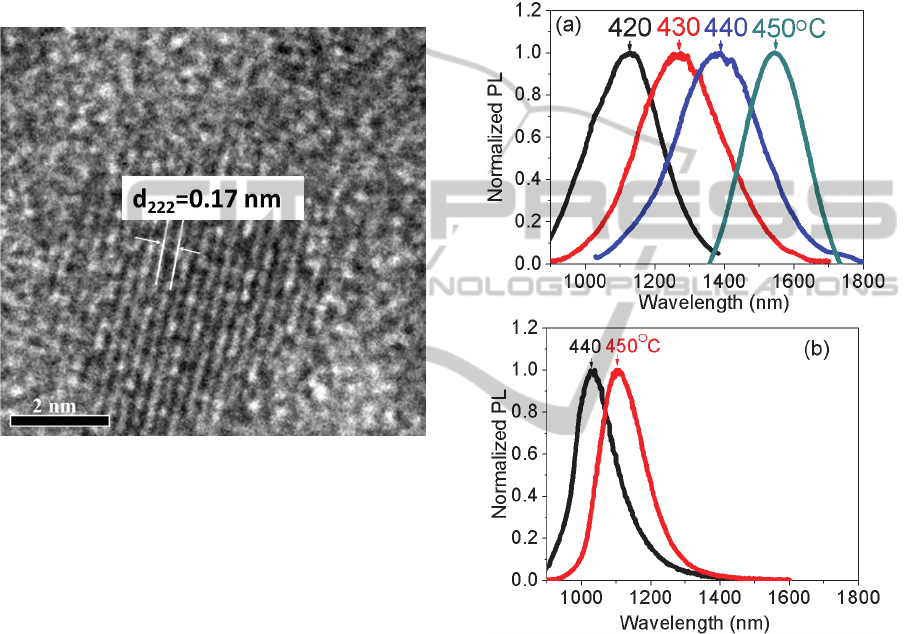
Heo, 2012b). The center wavelengths of PL were
~1000 and ~1100 nm at heat-treatment temperatures
of 440 and 450°C, respectively. It is obvious that
PbS QDs in glass surface coated with Ag paste
photoluminesced the longer wavelengths than did
those in unaffected regions. These results indicated
that the sizes of PbS QDs in Ag
+
migration regions
were larger than those in Ag
+
-free regions, which is
similar with the results from Ag
+
ion-exchange in
AgNO
3
solution or melt (Xu and Heo, 2012a, b).
Figure 2: TEM micrograph of a single PbS crystal. Glass
was subjected to Ag
+
ion migration at 320°C for 2 h and
heat treatment at 430°C for 10 h.
Another effect is that PbS QDs precipitated at
lower temperatures (420 and 430°C) after Ag
+
ion
migration. We did not observe any emission from
PbS QDs in Ag
+
-free glasses when heat-treatment
temperatures are less than 430°C (Figure 3b). But,
after Ag
+
ion migration, emissions from PbS QDs
were observed at heat-treatment temperatures of 420
and 430°C. This indicated that precipitation of PbS
QDs in glasses can be facilitated through Ag
+
ion
migration.
3.4 Ag NPs Promote the Formation of
PbS QDs in Glasses
At temperature of ~320°C, the neutral Ag
0
in paste
is oxidized to Ag
+
ions, which then diffuse into glass
(Najafi, 1992). During the heat treatment, Ag
+
ions
inside glasses are reduced to Ag
0
by capturing
electrons from impurities or non-bridging oxygens,
then aggregate to form Ag NPs (Wang, 1997). The
chemical states of silver in the glasses were
identified by XPS spectra. Figure 4 showed XPS
spectra of Ag
3d5/2
from the glass containing Ag
+
ions
after heat treatment (Xu and Heo, 2012b). The XPS
Ag
3d5/2
spectrum was separated into two peaks using
Gaussian curve fitting procedures. Results clearly
showed that the neutral Ag (Ag
0
) formed in glasses
after heat treatment, indicating the formation of Ag
NPs, but many Ag
+
ions still remained, probably
forming Ag
2
S or Ag
2
O crystals.
Figure 3: (a) Normalized PL spectra from glass surfaces
when glasses were subjected to Ag
+
ion migration at
320°C for 2 h and then heat treatment at 420, 430, 440 and
450°C for 10 h. (b) Normalized PL spectra from Ag
+
-free
glasses at heat-treatment temperatures of 440 and 450°C
for 10 h.
Ag NPs thus formed in glasses normally have an
absorption peak at wavelength of ~400 nm, but this
absorption peak was buried by PbS QDs in our
glasses. To confirm Ag NPs formed in our glasses
during the thermal treatment, we prepared a PbS
QDs-free glass with a nominal composition (mol %)
of 50SiO
2
- 35Na
2
O - 5Al
2
O
3
- 8ZnO - 2ZnS. The
glass was subjected to the same procedures as
before: Ag
+
ion migration at 320°C for 2 h by
coating Ag paste, then heat treatment at 400°C for
Solid-stateAg+IonMigrationfortheControlledPrecipitationofPbSQuantumDotsinGlasses
85
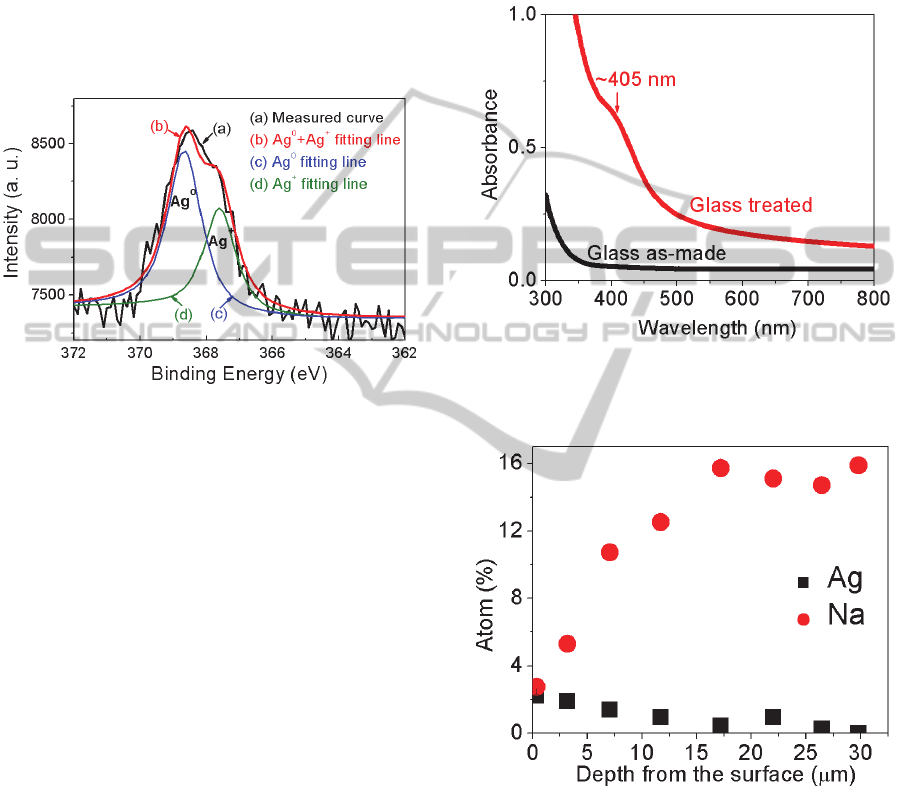
10 h to nucleate nanocrystals. A weak absorption
shoulder was observed at wavelength of ~405 nm
(Figure 5), which indicated that Ag NPs precipitated
in the glass. Thus, we assumed that Ag NPs were
also formed in glasses containing PbS QDs at this
low temperature of 400°C. During the initial stage of
heat treatment, Ag NPs quickly formed and provided
the nucleating sites for PbS QDs. Therefore, PbS
QDs precipitated at lower temperature, and grew
into the larger size after Ag
+
ion migration as we
observed in Figure 3a.
Figure 4: XPS spectra of Ag
3d5/2
from the glass containing
Ag
+
ions after heat treatment. Line (a) is measured curve,
and lines (b), (c) and (d) are results of the curve fitting by
assuming that the high binding energy component is due
to the neutral Ag (Ag
0
) while the low binding energy
component is from oxidized Ag
+
ions (Xu and Heo,
2012b).
EDX analysis showed that Ag
+
ions migrated
~30 μm into glass containing PbS QDs when it was
subjected to Ag
+
ion migration at 320°C for 2 h and
heat treatment at 430°C for 10 h (Figure 6).
Therefore, we believe that PbS QDs precipitated
within this ~30 μm layer in this glass.
3.5 Controlled Spatial Distribution of
Pbs QDs in Glasses
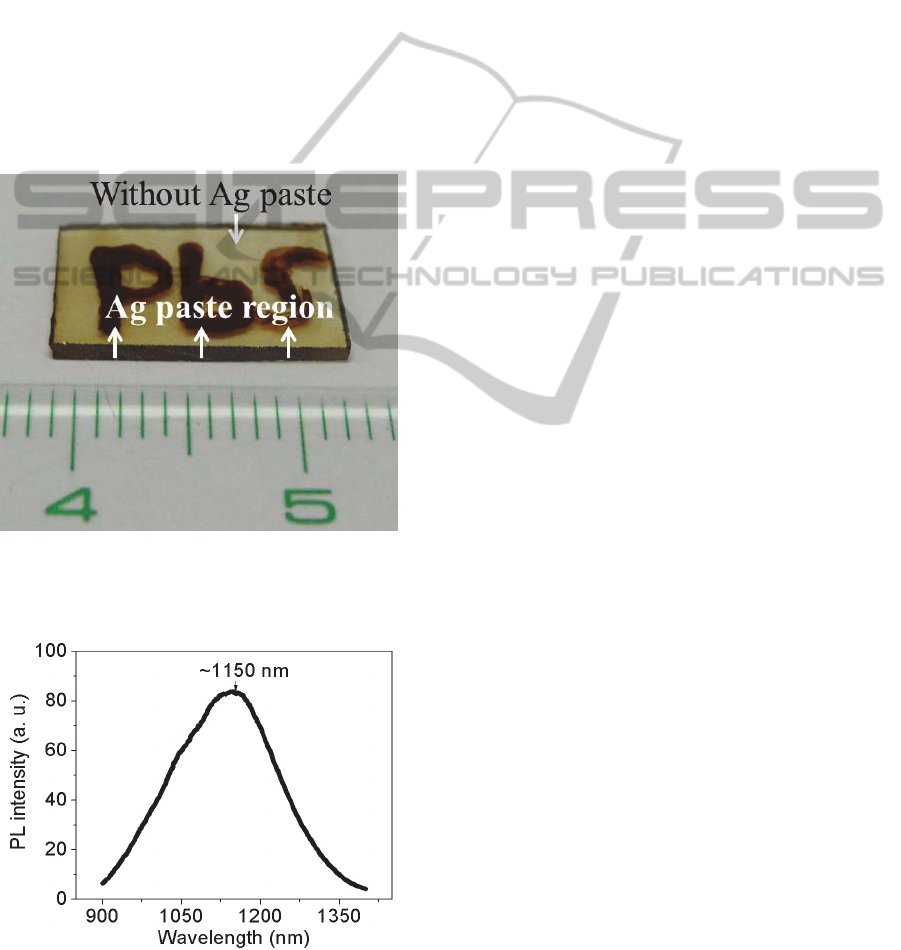
Ag paste with “PbS” word was coated on the glass
surface to evaluate the feasibility of controlling the
spatial distribution of PbS QDs in glasses.
Afterwards, sample was heat-treated at 320°C for 2
h for Ag
+
ion migration. After removing the Ag
paste, glass was heat-treated again at 420°C for 10 h
to nucleate PbS QDs. Photograph shows that the
word of “PbS” with dark brown appeared on the
glass surface where Ag paste was coated (Figure7).
PL spectrum from “PbS” word was also recorded as
shown in Figure 8. The center wavelength of PL was
~1150 nm, which is similar with the spectrum in
Figure 3a, and is from PbS QDs. Therefore, the
pattern of PbS QDs in glasses can be controlled by
simply control of Ag
+
ion inside glasses. Solid-state
Ag
+
migration could provide the more effective way
to control the spatial distribution of PbS QDs in
glasses, compared to ion implantation and
femtosecond laser irradiation techniques.
Figure 5: Absorption spectra of PbS QDs-free glass as-
made and the glass after Ag
+
migration at 320°C for 2 h
and then heat treatment at 400°C for 10 h.
Figure 6: Ag concentration along the cross-section of glass
by EDX analysis. Glass was subjected to Ag
+
ion
migration at 320°C for 2 h and heat treatment at 430°C for
10 h.
4 CONCLUSIONS
Solid-state Ag
+
ion migration and subsequent
thermal treatment were used to control the
precipitation of PbS QDs in glasses. After Ag
+
ion
migration and following heat treatment, PbS QDs
PHOTOPTICS2013-InternationalConferenceonPhotonics,OpticsandLaserTechnology
86
formed and were confirmed by TEM image. PbS
QDs in Ag
+
-migrated glass surface photoluminesced
the longer wavelengths than those in Ag
+
-free glass
when heat-treatment temperatures were 440 and
450°C. PbS QDs can also precipitate at temperatures
as low as 420 or 430°C after Ag
+
ion migration. Ag
concentration analyzed by EDX indicated that PbS
QDs could precipitate within ~30 μm layer from the
glass surface. XPS and optical absorption spectra
confirmed that Ag NPs formed at the initial stage of
heat treatment. Ag NPs thus formed provided the
nucleating sites and promote the formation of PbS
QDs in glasses. Solid-state Ag
+
ion migration
method can effectively control the spatial
distribution of PbS QDs in glasses, and it has the
potentials on space-selective formation of PbS QDs
in glasses for micro- or nano-photonic devices.
Figure 7: Photograph of PbS QDs with “PbS” word in
glass. Glass was subjected to Ag
+
ion migration at 320°C
for 2 h and then heat treatment at 420°C for 10 h.
Figure 8: PL spectrum from “PbS” word in Figure 7. Glass
was subjected to Ag
+
ion migration at 320°C for 2 h and
then heat treatment at 420°C for 10 h.
ACKNOWLEDGEMENTS
This work was supported by Basic Science Research
(2010-0022407), Priority Research Centers (2012-
046983) and World Class University (R31-30005)
Programs through the National Research Foundation
of Korea funded by the Ministry of Education,
Science and Technology.
REFERENCES
Borrelli, N. F. and Smith, D. W. (1994). Quantum
Confinement of PbS Microcrystals in Glass. J. Non-
Cryst. Solids, 180, 25-31.
Heo, J. and Liu, C. (2007). PbS Quantum-Dots in Glass
Matrix for Universal Fiber-Optic Amplifier. J. Mater.
Sci: Mater Electron., 18, S135-39.
Lamaestre, R. E., Majimel, J., Jomard, F. and Bernas, H.
(2005). Synthesis of Lead Chalcogenide Nanocrystals
by Sequential Ion Implantation in Silica. J. Phys.
Chem. B, 109, 19148-55.
Liu, C., Kwon, Y. K., Heo, J., Kim, B. H. and Sohn, I.
(2010). Controlled Precipitation of Lead Sulfide
Quantum Dots in Glasses Using the Femtosecond
Laser Pulses. J. Am. Ceram. Soc., 93, 1221-24.
Malyarevich, A. M., Yumashev, K. V. and Lipovskii, A.
A. (2008). Semiconductor-Doped Glass Saturable
Absorbers for Near-Infrared Solid-State Lasers. J.
Appl. Phys., 103, 081301- 25.
Najafi, S. I. (1992). Introduction to Glass Integrated
Optics. Artech House, Boston.
Stookey, S. D. (1959). Catalyzed Crystallization of Glass
in Theory and Practice. Ind. Eng. Chem., 51, 805-08.
Wang, P. W. (1997). Formation of Silver Colloids in
Silver Ion-exchanged Soda-lime Glasses during
Annealing. Appl. Surf. Sci., 120, 291-98.
Wise, F. W. (2000). Lead Salt Quantum Dots: the Limit of
Strong Quantum Confinement. Acc. Chem. Res., 33,
773-80.
Woggon, U., (1997). Optical Properties of Semiconductor
Quantum Dots. Springer, Berlin.
Xu, K., Liu, C., Dai, S., Shen, X., Wang, X. and Heo, J.
(2011). Influence of Silver Nanoclusters on Formation
of PbS Quantum Dots in Glasses. J. Non-Cryst. Solids,
357, 2428-30.
Xu, K. and Heo, J. (2012a). Lead Sulfide Quantum Dots in
Glasses Controlled by Silver Diffusion. J. Non-Cryst.
Solids, 358, 921-24.
Xu, K. and Heo, J. (2012b). Effect of Silver Ion-Exchange
on the Precipitation of Lead Sulfide Quantum Dots in
Glasses. J. Am. Ceram. Soc.,95, 2880-84.
Yong, K. T., Sahoo, Y., Choudhury, K. R., Swihart, M. T.,
Minter, J. R. and Prasad, P. N. (2006). Control of the
Morphology and Size of PbS Nanowires Using Gold
Nanoparticles. Chem. Mater., 18, 5965-72.
Solid-stateAg+IonMigrationfortheControlledPrecipitationofPbSQuantumDotsinGlasses
87
