
Automatic Detection of Skin Cancer
Current Status, Path for the Future
William V. Stoecker
1,2,3
, Nabin Mishra
1
, Robert W. LeAnder
4
, Ryan K. Rader
2,3
and R. Joe Stanley
1
1
Missouri University of Science And Technology, G20 Emerson Electrical Co. Hall, Rolla, MO 65409, U.S.A.
2
Stoecker & Associates, 10101 Stoltz Drive, Rolla, MO 65401, U.S.A.
3
University of Missouri School of Medicine, 1 Stadium Drive, Columbia, MO 65212, U.S.A.
4
Southern Illinois University Edwardsville, Campus Box 1801, Edwardsville, IL 62026, U.S.A.
Keywords: Machine Vision, Melanoma, Image Analysis, Color Processing, Dermoscopy, Skin Cancer.
Abstract: How far are we away from a Star-Trek-like device that can analyze a lesion and assess its malignancy? We
review the main challenges in this field in light of the Blois paradigm of clinical judgment and computers.
The research community has failed to adequately address several challenges ripe for the application of
digital technology: 1) early detection of changing lesions, 2) detection of non-melanoma skin cancers, and
3) detection of benign melanoma mimics. We highlight a new device and recent image analysis advances in
abnormal color and texture detection. Anthropomorphic paradigms can be applied to machine vision. Data
fusion has the potential to move automatic diagnosis of skin lesions closer to clinical practice. The fusion of
Blois’ high-level clinical information with low-level image data can yield high sensitivity and specificity.
Synergy between detection devices and humans can get us closer to this Star-Trek-like device.
1 OVERVIEW
The Machine as a Diagnostic Adjunct: Limiting the
Cognitive Span
1.1 Clinical Cognitive Span: The Blois
Paradigm
In the New England Journal of Medicine, Dr. M.
Scott Blois discussed the role of computers in the
clinic (Blois, 1980). The Blois paradigm states that
computers perform best using ‘low level’
information derived from physical or chemical
measurements, and perform worst using ‘high level’
information, such as patient statements. When a
doctor first sees a patient in the examination room,
there is a wide range of possible diagnoses.
Complicating the case is the interaction between
diagnoses that make the problem more complex.
Symptoms may be embellished or diminished. A
skilled clinician can adroitly navigate this subjective
information, separating benign conditions from
harmful conditions—the paramount diagnostic
challenge in medicine.
Figure 1: Blois Paradigm: Computers in the Clinic.
The complexity is high during the initial clinic
visit, where visual and verbal information is
unsorted and the range of possibilities, which Blois
termed the ‘cognitive span,’ is wide (point A, Figure
1). Further into the evaluation, we may have
chemical or physical information, e.g. blood samples
(point B, Figure 1). Humans function best with the
subjective information at point A, and computers
function best at point B, once the cognitive span is
narrowed. Where do we place image analysis in this
scheme, at point A, requiring human assessment, at
point B, or somewhere in between? With recent
developments, image analysis is still between points
A and B, but has moved closer to point B. In this
paper, we review several factors that have allowed
this advancement in computer image analysis. New
image analysis techniques, new data fusion
504
V. Stoecker W., Mishra N., LeAnder R., K. Rader R. and Stanley R..
Automatic Detection of Skin Cancer - Current Status, Path for the Future.
DOI: 10.5220/0004348605040508
In Proceedings of the International Conference on Computer Vision Theory and Applications (VISAPP-2013), pages 504-508
ISBN: 978-989-8565-47-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

techniques that combine clinical and image
information, and new uses of classifiers have
allowed advancements in the application of
computer vision that have increased computer
accuracy in diagnosing skin lesions.
1.2 Defining the Problem: Is This
Lesion a Skin Cancer, or Do I Have
a Skin Cancer Anywhere?
Most skin cancer detection research focuses on the
constrained problem: is this lesion a melanoma? In
Blois’ paradigm, at point A, the patient wants to
know: “Is there a skin cancer present anywhere on
my skin?” But research has been focused on the
narrower problem that is closer to point B: “Given a
single lesion, is this lesion a melanoma?” So we ask:
“Are there tools that could help us bridge this gap,
getting us from point A to point B?”.
For decades, total-body photography has been
used to assess the skin surface and detect changes
(Slue et al., 1988). A new tool called Melanoscan®
(Figure 2) eliminates the photographer and partially
automates image acquisition (Nguyen et al., 2010).
Figure 2: Melanoscan total bodyimages.
Melanoscan still requires the manual comparison
of images taken at different times to detect changes.
Recent advances in image registration can further
automate this process. We now have quantitative
information supporting Melanoscan’s effectiveness
in detecting melanomas at an earlier stage. During
the course of a study of melanoma in situ (Stricklin
et al., 2012), the Melanoscan clinic detected a higher
percentage of melanomas at the in situ stage (Figure
3). This represents progress toward answering the
more general question about having a melanoma
anywhere, with the possibility of detecting any
changes in skin cancer anywhere.
Figure 3: Melanoma in situ: invasive melanoma
(MIS:MM) ratio 2005-2009: 257 Total Lesions (Data from
(Stricklin et al., 2012)).
2 MELANOMA AND SKIN
CANCER DETECTION
2.1 Melanoma: Mankind’s ‘Cinderella
Cancer’
The societal burden of invasive melanoma is
significant. A measure of impact is the average
number of years of life lost (AYLL) caused by the
disease. The AYLL to melanoma is 23 years (Burnet
et al., 2005). Melanoma ranks 4th among all cancers
for AYLL/mortality (Salama et al., 2012), making it
one of four ‘Cinderella cancers,’ (along with brain,
uterine, and cervical) for which research, treatment,
and diagnostic advancements are significantly lower
than expected for the AYLL.
2.2 Importance of Non-Melanoma Skin
Cancer
To the societal burden of melanoma, we may add 3.5
million estimated cases of non-melanoma skin
cancer (NMSC) in the USA, that are annually
responsible for over 2,000 deaths per year (Bickers
et al., 2006). Economic costs of these skin cancers
exceed $2 billion in the USA, alone. Only scant
research has been done on automated NMSC
detection, which is now an area that could greatly
benefit from the effective application of computer
vision techniques (Guvenc et al., 2012); (Kefel et al.,
2012).
AutomaticDetectionofSkinCancer-CurrentStatus,PathfortheFuture
505
2.3 New Problem: Detecting a 2mm
Melanoma
During the 1990s, a group of researchers in Italy and
Austria gathered a large collection of dermoscopy
images of melanoma and benign lesions
(Argenziano et al., 2000). These advanced lesions
had features allowing high diagnostic accuracy in
automatic systems, with two reports showing 95-
96% diagnostic accuracy (Stanley et al., 2005),
(Wadhawan et al., 2011). Recently, the automatic
diagnosis problem has become more difficult with
earlier and smaller lesions, such as the 2mm
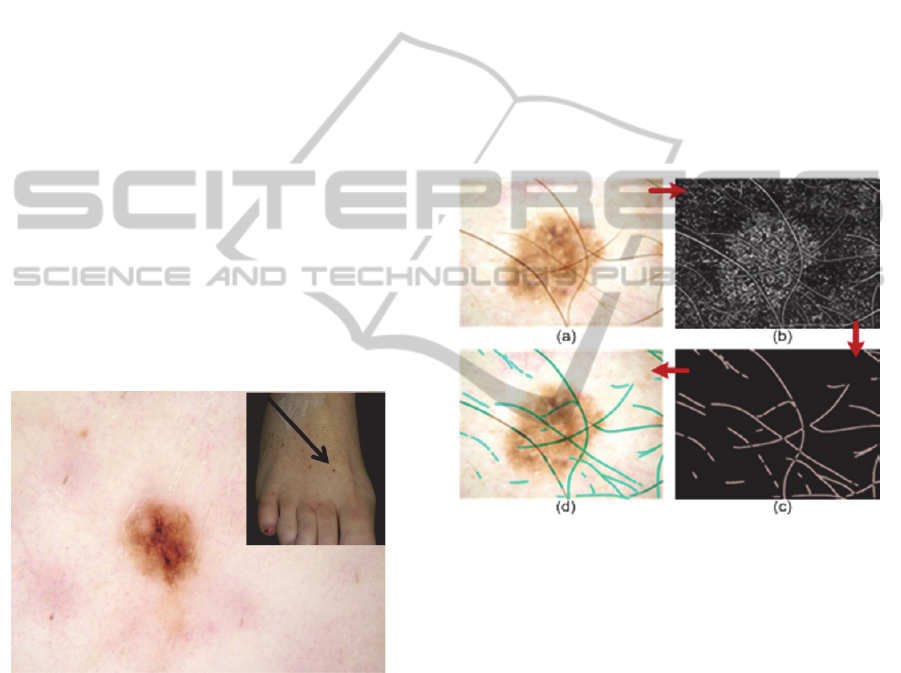
melanoma in a 48-year-old (Fig. 4).
2.4 Economic Burden of Benign
Lesions
In dermatology clinics, the most common tumor is a
benign lesion called a “seborrheic keratosis.” These
dark, fast-growing lesions alarm patients when they
first appear. Dermoscopy changes everything,
because it shows benign features with greater clarity.
Little research has been done to identify these
common lesions, which can be recognizing features
such as milia-like cysts (Stricklin et al., 2011).
Figure 4: 48y/o, 2mm melanoma on foot.
3 IMAGE BORDERS AND
ARTIFACTS
3.1 The Main Unsolved Computer
Vision Problems: Borders and
Artifacts
Automatic segmentation of skin cancer borders
would seem to be an easy task, yet the problem
remains unsolved. One leading technique applied to
these complex images is minimal energy contours
(Caselles et al., 1997). Hair removal, or hair
segmentation, is an essential part of image
processing, because hair mimics critical melanoma
features. Figure 5 shows an example of hair removal
from a dermoscopic image. The anisotropic
diffusion method of edge detection is employed to
accurately identify hair segments (Perona and Malik,
1990). Although this method is capable of
segmenting a majority of hairs, it is prone to
producing noise in the form of non-hair areas.
Morphological noise removal techniques are then
used to remove these non-hair segments.Figure 5:
(a) Original image, (b) Perona-Malik anisotropic
diffusion, (c) Hair mask after application of multiple
morphological noise removal techniques, (d) Hair
mask (cerulean) overlaid on original image.
Figure 5: (a) Original image, (b) Perona-Malik anisotropic
diffusion, (c) Hair mask after application of multiple
morphological noise removal techniques, (d) Hair mask
(cerulean) overlaid on original image.
4 ANTHROPOMORPHISM IN
IMAGE ANALYSIS
Innovation in computer vision can start with an
insight from human experience. Using the computer
vision technique “anthropomorphism,” we train the
computer to see objects that humans can see. To
detect amelanotic melanoma, the difficult variant
lacking pigment, we mimicked the observation of
Menzies, who noted that amelanotic melanoma has
more than one shade of pink (Menzies et al., 2008).
Yet the computers need a way to separate melanoma
pink from benign pink. We therefore analyzed a
different set of lesions—melanomas and benign
mimics having pink areas. We studied pink shade
VISAPP2013-InternationalConferenceonComputerVisionTheoryandApplications
506

and location variants, finding that location
outweighs shade. Using the anthropomorphic
finding that locations and shades of pink are
germane, we found varied shades of pink and used
the distance transform to overlay concentric
quintiles on these shades (Figure 6).
Figure 6: Automatically detected pink areas using 3-shade
analysis, lesion quintile map overlaid.
Calculating color, texture and blob features in
detected pink areas has yielded a high diagnostic
accuracy in preliminary research. Thus, the
anthropomorphic technique can provide useful
feature measurement for detecting skin cancer.
5 EARLIEST DETECTABLE
CHANGES IN MELANOMA:
ATYPICAL PIGMENT
NETWORK
An atypical pigment network (APN) is a critical
feature for successfully classifying melanoma.
Clinical APN presence yields an odds ratio of 9.0 for
melanoma (Argenziano et al., 2003). Figure 7 shows
the steps for automatic APN detection, which is used
as classifier inputs to predict malignancy. This
technique was successful in finding APN in the
2mm melanoma in Fig. 4.
6 DATA FUSION AND THE
BLOIS PARADIGM
The tiny melanoma presented earlier was diagnosed
when we added clinical information, specifically, the
patient’s concern and observation of lesion change.
A logistic regression analysis on 885 pigmented
lesions shows that the two features with the highest
Chi-square significance are clinical features: the
patient’s age and concern about the lesion, allowing
diagnosis of these images by clinical information
alone.
Figure 7: Melanoma in situ. (a) Original image, (APN
circled) (b) lesion mask, (c) relative red plane variance,
highest for granularity: red circles and blue oval (ruler
markings), (d) red variance mask after threshold, (e) mask
after threshold for green-to-blue ratio applied, (f) final
overlaid APN mask (green).
Data fusion using clinical and skin lesion image
information has been shown to improve lesion
discrimination by 19.9% over clinical and image
information only, while image features yield higher
lesion discrimination than clinical features by as
much as 9.7% (Cheng et al., 2012).
7 DIAGNOSTIC ASSISTANT IN
THE CLINIC
We have presented advances that further the goal of
automatic detection of skin cancer. The US Food
and Drug Administration noted the need to include
critical patient information in devices to maximize
diagnostic accuracy. Thus, even the early lesions
showing up more commonly in the clinic can now be
diagnosed. The path to success from research to
clinic should focus on the patient-centered problem,
“Do I have a skin cancer?” For the best patient
acceptance of automatic devices, assessment of
lesion change and non-melanoma skin cancers, as
well as benign melanoma mimics, should be
included in the research agenda of the computer
vision community.
AutomaticDetectionofSkinCancer-CurrentStatus,PathfortheFuture
507

REFERENCES
Argenziano, G., Soyer, H. P., De Giorgi, V., Piccolo, D.,
Carli, P., Delfino, M., Wolf, I. H., (2000). Interactive
atlas of dermoscopy CD: EDRA Medical Publishing
and New Media, Milan.
Argenziano, G., Soyer, H. P., Chimenti, S., Talamini, R.,
Corona, R., Sera, F., Kopf, A. W., (2003).
Dermoscopy of pigmented skin lesions: results of a
consensus meeting via the Internet. Journal of the
American Academy of Dermatology, 48(5), 679-83.
Bickers, D. R., Lim, H. W., Margolis, D., Weinstock, M.
A., Goodman, C., Faulkner, E., Dall T., (2006). The
burden of skin disease: 2004, a joint project of the
American Academy of Dermatology Association and
the Society for Investigative Dermatology. Journal of
the Academy of Dermatology, 55(3), 490-500.
Blois, M. S., (1980). Clinical judgment and computers.
New England Journal of Medicine, 303(4), 192-7.
Burnet, N. G., Jefferies, S. J., Benson, R. J., Hunt, D. P.
and Treasure, F. P., (2005). Years of life lost (YLL)
from cancer is an important measure of population
burden—and should be considered when allocating
research funds. British Journal of Dermatology, 92(2),
241-5.
Caselles, V., Kimmel, R., Sapiro, G. (1997). Geodesic
active contours, International Journal of Computer
Vision, 22(1): 61-79.
Cheng, B., Stanley, R. J., Stoecker, W. V., Stricklin, S.
M., Hinton, K. A. Nguyen, T. K., Moss, R. H. (2012).
Analysis of clinical and dermoscopic features for basal
cell carcinoma neural network classification. In press,
Skin Research and Technology.
Drugge, R. J., Nguyen, C., Gliga, L. and Drugge, E. D.
(2010). Clinical pathway for melanoma detection
using comprehensive cutaneous analysis with
Melanoscan. Dermatology Online Journal, 16(8), 1.
Guvenc, P., LeAnder, R. W., Kefel, S., Stoecker, W. V.,
Rader, R. K., Hinton, K. A., Moss, R. H. (2012) Sector
expansion and elliptical modeling of blue-gray ovoids
for basal cell carcinoma discrimination in dermoscopy
images. In press, Skin Research and Technology.
Kefel, S., Guvenc, P., LeAnder, R., Stricklin, S. M.,
Stoecker, W. V. (2012). Discrimination of basal cell
carcinoma from benign lesions based on extraction of
ulcer features in polarized-light dermoscopy images.
Skin Research and Technology, 18(4), 471-5.
Menzies, S. W., Kreusch, J., Byth, K., Pizzichetta, M. A.,
Marghoob, A., Braun, R, Johr, R. (2008).
Dermoscopic evaluation of amelanotic and
hypomelanotic melanoma. Archives of Dermatology,
144(9), 1120-7.
Perona, P. and Malik, J. (1990). Scale-space and edge
detection using anisotropic diffusion. In IEEE
Transactions on Pattern Analysis and Machine
Intelligence, 12(7), 629-39.
Salama, A. K., Rosa, N. D., Scheri, R. P., Herndon, J. E.,
Tyler, D. S., Marcello, J., Abernethy, A. P. (2012).
The effect of metastatic site and decade of diagnosis
on the individual burden of metastatic melanoma:
contemporary estimates of average years of life lost.
Cancer Investigation, 30(9), 637-41.
Slue, W., Kopf, A.W. and Rivers, J. K., (1988). Total-
body photographs of dysplastic nevi. Archives of
Dermatology, 124(8), 1239-43.
Stanley, R. J., Stoecker, W. V., Moss, R. H., Gupta, K.,
Jella, P. (2005). Color and structural features for
automatic skin lesion discrimination in dermoscopy
images. 6th World Congress on Melanoma,
Vancouver, BC, September 7, 2005.
Stricklin, S. M., Stoecker, W. V., Malters, J. M., Drugge,
R., Oliviero, M. & Rabinovitz, H. S., (2012).
Melanoma in situ in a private practice setting 2005
through 2009: Location, lesion size, lack of concern.
Journal of the American Academy of Dermatology,
67(3), e105-9.
VISAPP2013-InternationalConferenceonComputerVisionTheoryandApplications
508
