
The Use of Timing Control Strategies to Overcome Severe Time
Constraints during Rapid Interception
Tetsuya Ijiri, Masahiro Shinya, Kohtaroh Hagio
and Kimitaka Nakazawa
Department of Life Sciences, Laboratory of Sports Sciences, The University of Tokyo, Komaba, Tokyo, Japan
Keywords: Baseball, Visuomotor Coordination, Startle Reaction, Acoustic Stimulation, Sports, Vision, Feedback,
Feedforward, Efference Copy, Electromyography (Emg), Excitability, Subcortical, Motor Circuit.
Abstract: We investigated the mechanisms underlying timing of rapid interceptive actions under severe time
constraints, such as those required in baseball, cricket, and tennis. To compensate for the temporal
uncertainty of a moving target, participants were required to control their movement onset and/or duration.
In Experiment 1, we tested how movement onset and/or duration are controlled under severe time
constraints in a rapid baseball-simulation interceptive task. We found two distinct control strategies that
modulated task performance. We also found that corrections to ongoing movements occurred more rapidly
than had previously been reported. In Experiment 2, we used startling acoustic stimulation to investigate the
detailed mechanisms underlying decisions about the timing of movement onset. Our findings indicate that
the timing of movement onset is modified continuously via a subcortical motor circuit. Overall, our findings
indicate that rapid movement decisions rely on a hybrid of feedforward and feedback control, allowing for
the circumvention of severe time constraints during rapid interceptive actions.
1 INTRODUCTION
Elite athletes exhibit extremely high spatiotemporal
accuracy during rapid interceptive action, such as the
movements required to hit a moving ball in baseball,
cricket, or tennis. In these sports, a ball may travel
from its origin to the hitting point in less than half a
second, and the hitting action takes approximately
200 ms (Gray, 2002a). Opponents attempt to
maximize the spatial and temporal uncertainty, and
so both the ball speed and trajectory are highly
unpredictable. Despite these challenges, professional
players are able to hit a ball with a spatiotemporal
accuracy in the range of centimetres and
milliseconds (Regan, 1992). To achieve a high level
of accuracy in the timing of interceptive actions,
both movement onset and duration must be precisely
controlled.
Accurate control of movement onset and/or
duration is difficult under the above-mentioned
conditions because of the relatively long
physiological delay required for processing sensory
information. Visuomotor delay (VMD), which is the
time period between a visually detectable event and
the resulting observable response to the event, has
been reported to range from 100 to 300 ms (Runigo
et al., 2010); (Runigo et al., 2005); (Bootsma and
Van Wieringen, 1990). This delay presents a
challenge when making online corrections to one’s
swing duration under severe time constraints.
It is also difficult to pinpoint the exact onset of a
movement command using visual information about
a moving target. This is because motor commands
are triggered by visual stimulus events that occur
approximately 150 ms before movement onset
(Marinovic et al., 2009) and there is no enough time
for discriminating the difference of ball speed.
Although players utilize opponent movements (See
Müller and Abernethy, 2012 for a review) and
knowledge about prior trial (Gray, 2002a); (Gray
2002b) to anticipate ball trajectory and speed, they
are still at risk of incorrectly anticipating a
movement resulting high demands of online
correction. The mechanism that permits the
circumvention of such time constraints remains
unclear.
The main purpose of this study was to examine
the control mechanisms underlying the timing of
rapid interceptive actions, such as those that allow
athletes to circumvent severe time constraints and
achieve high temporal accuracy. We conducted two
experiments wherein participants performed a
5
Ijiri T., Shinya M., Hagio K. and Nakazawa K..
The Use of Timing Control Strategies to Overcome Severe Time Constraints during Rapid Interception.
DOI: 10.5220/0004614100050012
In Proceedings of the International Congress on Sports Science Research and Technology Support (icSPORTS-2013), pages 5-12
ISBN: 978-989-8565-79-2
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
baseball-simulation rapid interceptive task, with
faster and slower balls presented in a random order.
In Experiment 1 we investigated the efficacy of
strategies for controlling timing during rapid
interception relative to task performance. We
compared our experimental results with data
regarding batters in actual baseball games, which
had been recorded with a high speed camera. In
Experiment 2 we used startling acoustic stimulation
to examine the specific mechanisms that enable an
individual to overcome the severe time constraints
and plan their swing onset in response to various ball
velocities (Carlsen et al., 2011); (Valls-Solé et al.,
1999). This technique allowed us to investigate the
temporal course of motor preparation.
2 EXPERIMENT 1
2.1 Materials and Methods
2.1.1 Participants
Twenty six healthy young males participated in the
experiment (age range 18-24; mean = 20 years). All
participants reported minimal experience with fast
ball sports like baseball, cricket, or tennis, and stated
they were right handed and had normal or corrected-
to-normal vision. Ethical approval for this study was
granted by the Ethical committee of The University
of Tokyo and all participants provided informed
consents.
2.1.2 Task and Apparatus
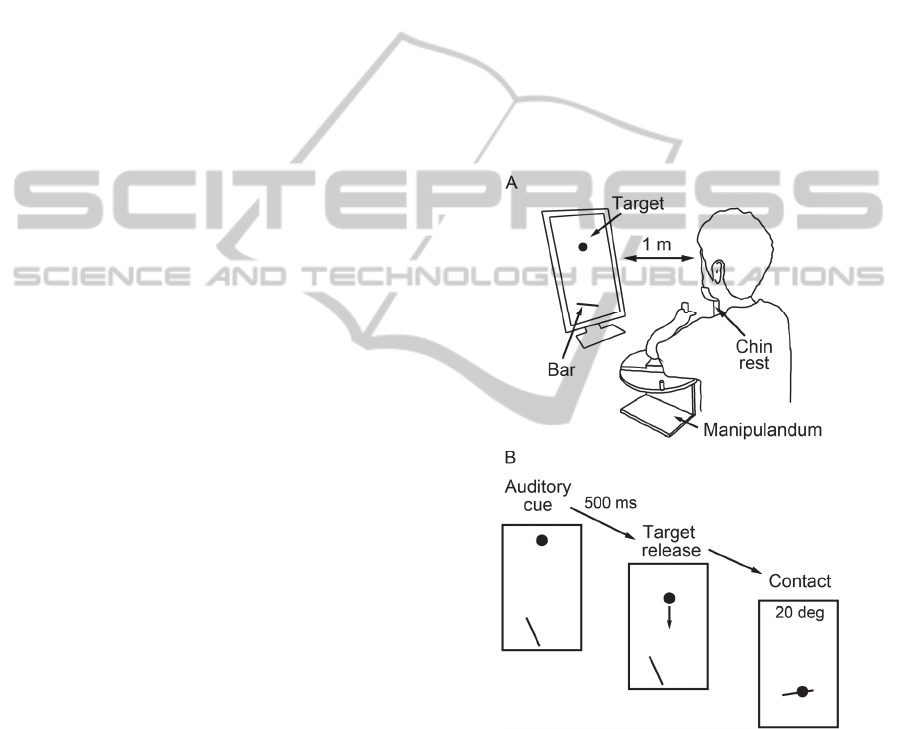
The experimental setup was shown in Figure 1. The
participants were asked to intercept a moving virtual
ball on a computer screen (23.6 inches, 1920 × 1080
pixels and a refresh frequency of 120 Hz) using a
virtual arm that was controlled by the actual
movement of their left elbow joint. Participants sat
on a chair and placed their left forearm on a
manipulandum. Movement of the manipulandum
was calibrated such the degrees of rotation matched
that of the virtual bat. A line that was horizontal to
the axis of bat rotation was defined as the optimal hit
point, and participants were encouraged to hit the
ball at that point. When the participants set the bat at
the initial position (e.g. -65 degrees from the optimal
hit point), an auditory warning cue was given. After
500 ms, the ball was released downward. The
participants were instructed to fully extend their
elbow and to not stop the bat at the optimal hit point.
2.1.3 Procedures
The participants were exposed to two paired-speed
conditions; 'Slow or Medium' and 'Medium or Fast',
in which ball speeds varied between trials. Time-to-
contact (TTC) was defined as the interval from ball
release to the arrival of the ball at the optimal hit
point. The TTC for the different conditions were 670
ms (Slow), 540 ms (Medium), and 410 ms (Fast).
Participants completed 24 trials in each set and 4 sets
in total for each condition. The control strategies
used by each participant became stable in the second
half of the 4 sets, and so the last 2 sets were regarded
as the test sessions and included in the analysis. All
the computerized events were controlled by a
program written with LabVIEW software (National
Instruments).
Figure 1: Experimental setup. (A) Physical set up. Using a
manipulandum system, participants control the rotation of
virtual bar projected of the monitor. (B) Virtual setup and
time course of a trial. An auditory cue was provided,
followed by 500 ms of foreperiod. The degree of the bar at
the contact with the target was provided after every trial
combined with visual feedback of the bar angle.
2.1.4 Data Reduction
Data were analysed offline using MATLAB
(Mathworks) software and JMP10 (SAS Institute,
NC, USA). The elbow angle data were digitally low-
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
6
pass filtered with a fourth-order, zero-phase-lag
Butterworth filter at a cut-off frequency of 8 Hz.
To analyse the difference in control strategies
between participants, we calculated swing onset and
swing duration for each trial. The swing onset was
defined as the time from ball release to the moment
at which the angular velocity of the bat had reached
30 degrees/s and remained constant or surpassed this
velocity for an additional 50 ms. The swing duration
was defined as the time from swing onset to the
moment at which the bat angle reached the optimal
hit point. We also calculated delta onset, which was
defined as the mean difference in swing onset
between the faster and slower ball speeds. In
addition, we calculated the delta duration, which was
defined as the mean difference in swing duration
between the faster and slower ball speeds.
To evaluate task performance, we analyzed
constant error (CE) and variable error (VE) as
indices of error direction and variability, respectively.
The CE was defined as the difference between the
TTC of the ball and the time at which the bat
reached the optimal hit point (i.e. the sum of the
swing onset and swing duration).
2.1.5 Recording Systems
The elbow angle data were measured using a
potentiometer attached to the joint of the
manipulandum. Electromyographic (EMG) signals
were recorded via double differential surface
electrodes (DE-3.1, Delsys) placed on the biceps
brachii and triceps brachii of the left arm. The EMG
signals were amplified (gain: 1000) using an EMG
amplifier (BAGNOLI-8, Delsys). All data were
digitally sampled at 1000 Hz using a program
written with LabVIEW software.
2.1.6 Baseball Game Data
We recorded two baseball games: one at a university
and one at a high school national tournament. Data
were collected using a camera (Exilim EX-F1, Casio,
Japan) with a frame rate of 600 fps. The camera was
placed approximately 20 meters behind the batter
and captured both the batter and pitcher in the same
frame. We analysed a total of 41 trials, or instances
where the batter swung at the ball, at the university
game. At the high school game, we analysed a total
of 39 trials.
The timing of ball release, swing onset, contact
of the bat with the ball, and TTC of the pitched ball
were analysed using image analysis software
(MediaBlend, Japan). The timing of ball release and
the contact point were easily detected by visual
inspection. The timing of swing onset was defined as
the time point at which a successive downward
movement of the batter’s hands was detected. TTC
was defined as the time between the ball release and
the contact between the ball and bat. To assess
whether batters had a tendency to change their swing
onset and/or swing duration according to perceived
ball speed, we calculated Pearson's correlation
coefficient between the TTC and the two variables.
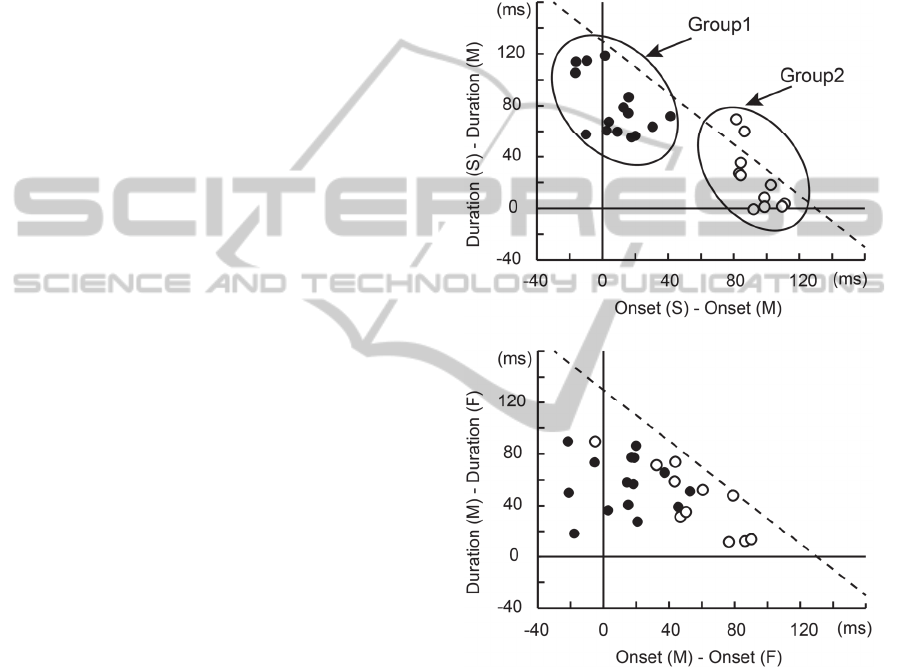
Figure 2: The distribution of delta onset and delta duration
in slow or medium condition (top) and medium or fast
condition (bottom) Diagonal dashed line represents
optimal compensation of the 130 ms gap of TTCs. Control
strategy in slow or medium condition was divided into two
groups (filled circle: group1, open circle: group2). Similar
tendency was observed in medium or fast condition.
2.2 Results
2.2.1 Different Control Strategies between
Participants
The distribution of the delta onset and delta duration
for all participants is shown in Figure 2. Using the
Shapiro-Wilk normality test, we found that the
TheUseofTimingControlStrategiestoOvercomeSevereTimeConstraintsduringRapidInterception
7
distribution of the delta onset in the ‘Slow or
Medium’ condition was not normal distribution (W
= 0.87, p < 0.001). Therefore, we divided the
participants into two subgroups, as shown in the top
panel of Figure 2. This tendency was also observed
in the 'Medium or Fast' condition, as shown in the
bottom panel of Figure 2. We compared the timing
accuracy between these two groups.
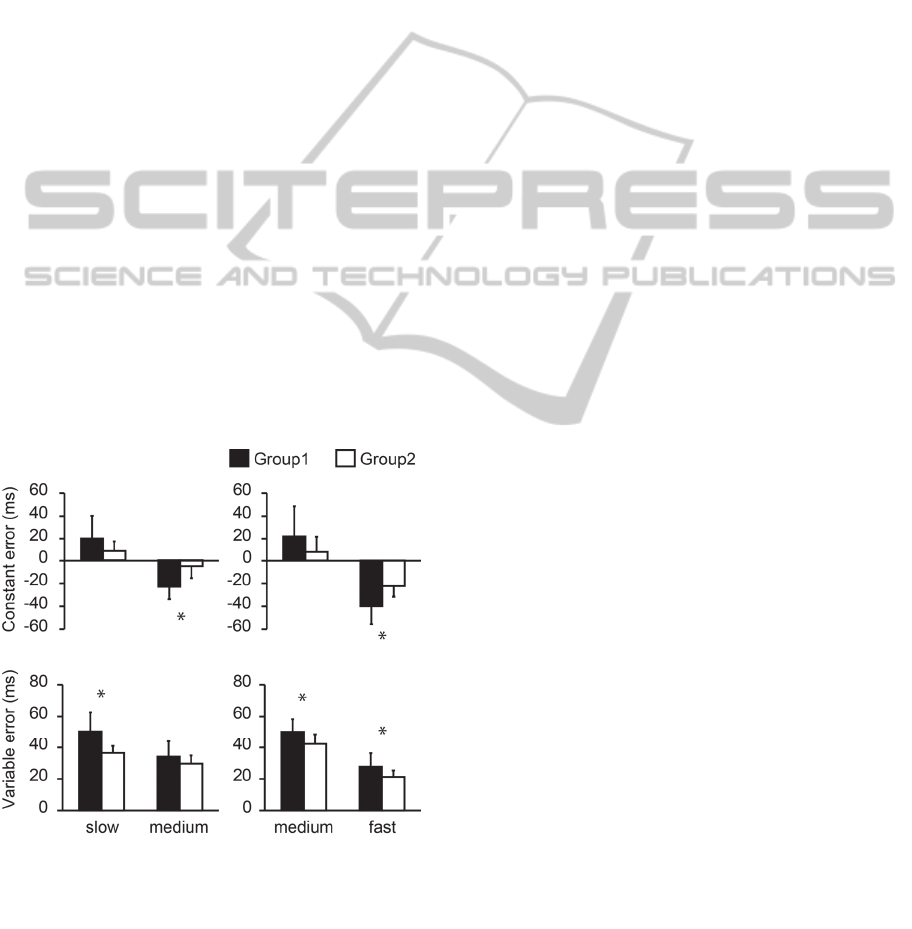
2.2.2 Timing Accuracy
We compared the CE and VE of ball speed between
the two groups using Welch's t-test. The significance
level was set at 0.05 (fig. 3). The CE of group 1 was
significantly higher than that of group 2 for the
medium speed in the ‘Slow or Medium’ condition (t
= 4.33, p < 0.001) and the fast speed in the ‘Medium
or Fast’ condition (t = 3.59, p < 0.001). No
significant differences were found in the other
conditions (the slow speed in the 'Slow or Medium
condition'; t = 1.99, p = 0.059, the medium speed in
the 'Medium or Fast condition'; t = 1.78, p = 0.089).
The VE of group1 was significantly larger than
that of group 2 for the slow speed in the ‘Slow or
Medium’ condition (t = 4.06, p < 0.001) and in both
speeds in the ‘Medium or Fast’ condition (Medium; t
= 2.78, p = 0.011, Fast; t = 2.50, p = 0.021). There
was no significant difference in the medium speed in
the 'Slow or Medium' condition (t = 1.44, p = 0.16).
Figure 3: Constant error and variable error in paired-speed
condition (filled bars: group1, open bars: group2).
*p<0.05s, significant difference between groups. The error
bars refer to ± 1SD.
2.2.3 EMG Latency for Online Correction
The participants in group 1 mainly modulated swing
duration and not swing onset. We used EMG data to
investigate the detailed mechanisms underlying
online corrections in movement under the severe
time constraint. Differences in control strategies
were reflected in triceps brachii activity but not
biceps brachii activity, so we analysed only the
EMG data for triceps brachii. We sought to evaluate
the time required to correct ongoing swing speed.
The latency for online correction was defined as the
time point of the first deviation from the averaged
EMG amplitude between the faster and slower ball
speeds, as shown in the top panel of Figure 4. To
guide this measure, we calculated the time at which a
significant difference in amplitude was observed for
at least 15 ms. This was established using a
successive t-test (p<0.05) that compared the
averaged EMG amplitude of the two speeds. We also
analysed the EMG onset in each trial, which was
defined as the time point at which EMG activity
increased by more than 3 SDs above baseline levels
(the mean level during 100 ms of EMG activity
collected before ball release).
The correction latency in the 'Slow or Medium'
condition was 246.2 ± 16.7 ms, and the average
EMG onsets in Group 1 for the slow speed and
medium speed were 172.2 ± 76.6 ms and 174 ± 56.8
ms. The correction latency in the 'Medium or Fast'
condition was 210.9 ± 20.8 ms, and the average
EMG onsets in Group 1 for the medium speed and
fast speed were 142.1 ± 69.2 ms and 135.5 ± 52.7 ms
(bottom panel of fig. 4). Note that the time between
EMG onset and the correction latency was
approximately 70 ms in all conditions, and this value
was much smaller than previously reported VMD.
This suggests the involvement of internal feedback
loops that integrate efferent and afferent signals
(Wolpert et al. 1995) with negligible delay
(discussed in the following section).
2.2.4 Behaviours of Baseball Batters
The correlation coefficient between swing onset and
TTC was 0.82 (p < 0.001) and between swing
duration and TTC was 0.53 (p < 0.001) in the high
school game. In the university game, the correlation
coefficient between swing onset and TTC was 0.60
(p < 0.001) and between swing duration and TTC
was 0.29 (p = 0.06).
3 EXPERIMENT 2
In Experiment 1, the timing strategy for changing
swing onset outperformed the strategy for changing
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
8

swing duration. Moreover, we were able to speculate
about a mechanism that makes the correction of
ongoing movement possible. However, the
mechanism involved in modulating swing onset was
still unclear. In Experiment 2, we sought to
investigate the detailed control mechanisms involved
in changing swing onset to adjust to different ball
speeds.
Previous studies have reported that corticospinal
excitability, measured using single-pulse transcranial
magnetic stimulation (TMS), increases about 100 ms
before EMG onset (Starr et al. 1988; McMillan et al.
2004). Although this excitatory drive (Floeter &
Rothwell 1999) is modulated by a cortical inhibitory
control mechanism (Nakamoto and Mori, 2012);
(Reynolds and Ashby, 1999); (Soto et al., 2010),
other inhibitory mechanisms involving subcortical
motor circuits have been suggested (Maslovat et al.,
2012); (Soto et al., 2010).
Startling acoustic stimuli (SAS) is a useful probe
for pre-programmed motor commands and has been
used to investigate the temporal course of motor
preparation and subcortical motor circuit excitability.
If a motor command is not prepared in advance (e.g.
in a choice reaction time task), SAS does not
facilitate any voluntary response relative to the task
(Carlsen et al., 2004). However, in a simple reaction
time task in which a motor command can be
prepared in advance, SAS can elicit a voluntary
response with a very short latency (Valls-Solé et al.,
1999). In an anticipation-timing task, motor
preparation occurs as late as 200 ms before response
time (Carlsen and Mackinnon, 2010); (Carlsen et al.,
2008).
We hypothesized that participants who
predominantly changed their mainly swing onset in
paired-speed condition would prepare a motor
command and exhibit increased subcortical motor
circuit excitability relative to faster ball speeds,
regardless of actual ball speeds. If ball speed was
slow, this subcortical motor circuit would be
inhibited so as to prevent a motor command from
being inaccurately timed. This would delay the
''deadline'' for decision making about speed
discrimination, resulting in the circumvention of
severe time constraints.
3.1 Materials and Methods
3.1.1 Participants
Six healthy male volunteers participated in the
experiment (ages: 25.5 ± 1.5 years). All participants
were right-handed, had normal or corrected-to-
normal vision, and provided informed consent.
3.1.2 Task and Apparatus
The experimental task, apparatus, and recording
methodology were identical to those in Experiment 1
except that a loud speaker (DSR 112, YAMAHA,
Japan) was placed 50 cm behind the participants’
heads. SAS was generated by a customized program
written using LabVIEW software that produced
broadband white noise (duration; 50 ms, rise time; 1
ms). The signal was amplified and presented at an
intensity of 123±1 dB through the loudspeaker.
EMG signals were obtained from electrodes placed
on the triceps brachii (TB), biceps brachii (BB), and
sternocleidomastoid (SCM). SCM activity was
regarded as an indication of startle response.
3.1.3 Procedures
Experiment 2 consisted of two conditions; a paired-
speed condition and a single-speed condition. The
TTC in the paired-speed condition was Slow (800
ms) and Fast (500 ms), whereas the TTC in the
single-speed condition were solely Slow (800 ms).
We set the Slow TTC larger than in Experiment 1 to
make changing one’s swing onset relatively easy and
thus ensure stable task performance.
Prior to the experimental session, participants
performed a practice session in which they stabilised
their timing strategies. The SAS was not presented in
the practice session. Swing duration and bat angle at
the moment of contact were provided as a feedback
for each trial. All participants completed between 60
and 90 practice trials. Data from participants who
mainly changed their swing duration were discarded
because the aim of Experiment 2 was to investigate
the detailed mechanisms involved in changing one’s
movement onset.
Following the practice session, each participant
performed a total of 80 experimental trials.
Participants were instructed to use the same strategy
and swing duration as in the practice trials. In 8
Slow-speed trials (10% of all trials), the SAS was
presented 150 ms after the moment of ball release.
Note that the SAS was not presented in the Fast-
speed trials.
Participants then performed 20 practice trials in
the single-speed condition without the presentation
of SAS. Finally, participants completed 80
experimental single-speed trials. In 8 trials, the SAS
was presented 150 ms after the moment of ball
release.
TheUseofTimingControlStrategiestoOvercomeSevereTimeConstraintsduringRapidInterception
9
3.1.4 Data Reduction
EMG onset was analysed using the same algorithm
as Experiment 1. The probability of startle response
elicited by SAS was analysed with respect to SCM
activity to evaluate the excitability of the subcortical
motor circuit (Maslovat et al., 2012). SCM activity
that occurred within 120 ms of the SAS presentation
was regarded as a startle reflexive response.
Similarly, the probability that a preprogrammed
motor command had been triggered early was
analysed in terms of TB activity to assess the state of
advance motor preparation. EMG activity at the TB
that occurred within 150 ms of the SAS presentation
was regarded as an early release of preprogrammed
motor command.
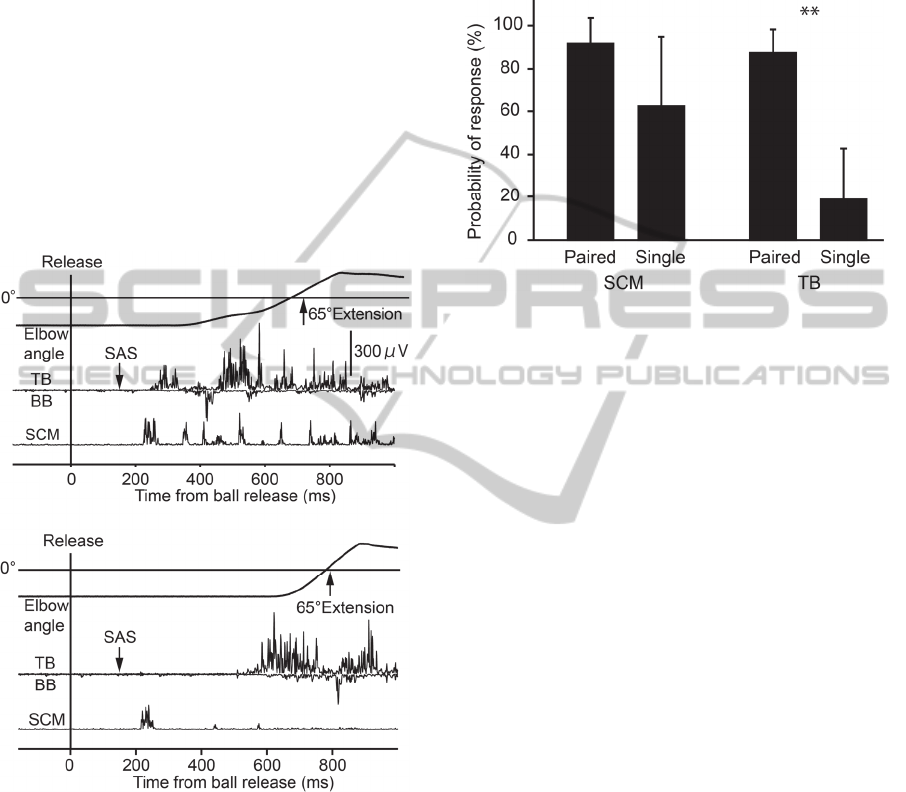
Figure 5: Typical EMG and elbow angle data from startle
trials in paired-speed (top) and single-speed condition
(bottom). SAS elicited both TB and SCM activities in
paired-speed condition, but elicited only SCM activity in
single-speed condition.
3.2 Results
3.2.1 Behaviour in Non-startle Trials
Swing durations in the non-startle trials were
223.6±24.8 ms for the Slow-speed trials and
184.5±15.5 ms for Fast-speed trials in paired-speed
condition. The EMG onsets in the non-startle trials
were 482.7±25.4 ms for the Slow-speed trials and
280.1±17.7 ms for the Fast-speed trials. These
results indicate that SAS was presented 332.7 ms
(for the Slow-speed condition) and 130.1 ms (for the
Fast-speed condition) prior to TB activity onset.
Figure 6: Probability of response in SCM and TB in paired
and single-speed condition. **p<0.01, significant
difference in the value between paired and single-speed
condition. The error bar refer to ±1 SD.
3.2.2 Response Probability of Startle
Indicator
Typical responses in the startle and non-startle trials
are illustrated in Figure 5. SAS in the paired-speed
condition typically elicited early activity in both the
SCM and TB, whereas SAS in the single-speed
condition evoked activity in the SCM but not the TB.
The probability of a startle response elicited in
the SCM by SAS was 94.4±10.1 % in the paired-
speed condition and 75.0±31.6 % in the single-speed
condition (left panel of Figure 6). A paired sample t-
test did not reveal a significant difference between
the response probability for the paired and single-
speed conditions (t = 1.56, p = 0.18).
3.2.3 Probability and Latency of Early
Release of Prepared Motor Command
The probability of early triggering of a prepared
motor command in the TB was 86.1±8.6 % in the
paired-speed condition and 16.7±20.4 % in the
single-speed condition (right panel of Figure 6). A
paired sample t-test revealed a significant difference
between the probability of TB response in the paired
and single-speed conditions (t = 7.47, p < 0.01).
The EMG onset of early triggered TB activity in
the paired-speed condition was 239.5±11.3 ms (89.5
ms from SAS presentation).
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
10

4 DISCUSSION
4.1 Differences in Control Strategy
In Experiment 1, we found two main timing
strategies. In group 1, participants mainly modulated
their movement duration according to the speed of
the ball, whereas in group 2, participants modulated
their movement onset according to the speed of the
ball with a fixed movement duration. However,
participants did not exclusively control either their
swing onset or swing duration. Rather, both swing
onset and swing duration were flexibly modified,
and the balance between these two variables was
different among participants. In our observations of
batters in high school baseball games, we found a
significant correlation between both swing onset and
TTC and between swing duration and TTC,
indicating that these two variables are flexibly
controlled.
The data from the present study are not sufficient
to speculate about what distinguishes the two
observed control strategies. However, we presume
that individuals who mainly change their swing
duration take relatively longer to discriminate ball
speed than those who emphasise swing onset.
4.1.1 Differences in Task Performance
The task accuracy in group 2 was higher than that of
group 1. Reasons for this difference might include
the number of control variables involved in online
correction and the short time period available for the
correction. To correct an ongoing movement, the
timing of correction and modified movement speed
need to be considered together. On the other hand,
only accurate timing of movement onset is required
to change swing onset. Moreover, the time available
for online correction was minimal given the time
constraints in this study, even if it were possible to
correct ongoing movement with a short delay
(discussed in the next section).
4.1.2 Latency for Online Correction
The participants in Group 1 started their swing at the
time required to accommodate faster ball speeds and
modified their swing speed to adjust to slower ball
speeds. The correction latency was about 70 ms from
EMG onset in both conditions. This is much shorter
than previously reported VMD values, which range
from 100 to 300 ms (Runigo et al., 2010); (Runigo et
al., 2005); (Bootsma and Van Wieringen 1990), but
is comparable to a latency ranging from 83 to 122
ms reported by Higgins and Angel (1970) and 30 to
150 ms reported by Cooke and Diggles (1984).
Therefore, the corrective response observed in this
study can be accounted for not by sensory feedback
loops but internal feedback loops (Wolpert et al.,
1995). A forward model in the loops provides a
reliable estimation of effector location and velocity
by integrating efferent and afferent signals with
negligible delays, and makes online correction
possible for rapid and short movements (see
Desmurget and Grafton, 2000 for a review). The
observed correction latency in the present study
indicates the contribution of internal feedback loops
in the control of rapid interceptive movements.
4.1.3 Effect of SAS on Voluntary Response
In Experiment 2, SAS was presented in the slow-
speed trials in both paired and single-speed
conditions. However, the SAS consistently elicited
TB activity in the paired-speed but not the single-
speed condition (fig. 6). The timing of SAS
presentation was 332.7 ms prior to EMG onset of TB
in the slow-speed trial. Previous studies have
reported that motor preparation is not complete until
less than 200 ms before response time (Carlsen and
Mackinnon, 2010); (Carlsen et al., 2008). Our
participants appear to have prepared motor
commands with respect to the timing of a fast-speed
ball before discriminating the actual ball speed.
When the ball speed is slow, subcortical motor
circuit excitability might be inhibited so as to
prevent a motor command from being inaccurately
timed. We did not randomize the experimental order
of the paired and single-speed conditions, and so did
not eliminate the possible effect of habituation to the
SAS (Maslovat et al., 2012). Further study is needed
to clarify the influence of this confounding factor.
5 CONCLUSIONS
In summary, we have shown that a timing strategy in
which both movement onset and duration were
controlled outperformed a strategy in which
movement duration was mainly modulated with less
of an emphasis on onset. The rapid correction of
ongoing movement likely involves internal feedback
loops. Moreover, using startle acoustic stimulation,
we have shown that modulation of excitability in
subcortical motor circuits is likely involved in the
continuous control of movement onset under severe
time constraints.
TheUseofTimingControlStrategiestoOvercomeSevereTimeConstraintsduringRapidInterception
11

REFERENCES
Bootsma, R. J. & Van Wieringen, P. C., 1990. Timing an
attacking forehand drive in table tennis. Journal of
Experimental Psychology: Human Perception and
Performance, 16(1), pp.21–29.
Carlsen, A. N. et al., 2011. Considerations for the use of a
startling acoustic stimulus in studies of motor
preparation in humans. Neuroscience and
biobehavioral reviews, 35(3), pp.366–76.
Carlsen, A. N. et al., 2008. Motor preparation in an
anticipation-timing task. Experimental brain research,
190(4), pp.453–61.
Carlsen, A. N., Chua, R. & Inglis, J., 2004. Can prepared
responses be stored subcortically? Experimental brain
research, 159(3), pp.301–9.
Carlsen, A. N. & Mackinnon, C. D., 2010. Motor
preparation is modulated by the resolution of the
response timing information. Brain research, 1322,
pp.38–49.
Cooke, J. D. & Diggles, V. A., 1984. Rapid error
correction during human arm movements: evidence for
central monitoring. Journal of motor behavior, 16(4),
pp.348–63.
Desmurget, M. & Grafton, S., 2000. Forward modeling
allows feedback control for fast reaching movements.
Trends in cognitive sciences, 4(11), pp.423–431.
Floeter, M. K. & Rothwell, J. C., 1999. Releasing the
brakes before pressing the gas pedal. Neurology, 53(4),
pp.664–664.
Gray, R., 2002a. Behavior of college baseball players in a
virtual batting task. Journal of Experimental
Psychology: Human Perception and Performance,
28(5), pp.1131–1148.
Gray, R., 2002b. “Markov at the Bat”: A Model of
Cognitive Processing in Baseball Batters.
Psychological Science, 13(6), pp.542–547.
Higgins, J. R. & Angel, R. W., 1970. Correction of
tracking errors without sensory feedback. Journal of
experimental psychology, 84(3), pp.412–6.
Marinovic, W., Plooy, A. M. & Tresilian, J. R., 2009. The
utilisation of visual information in the control of rapid
interceptive actions. Experimental psychology, 56(4),
pp.265–73.
Maslovat, D., Carlsen, A. N. & Franks, I. M., 2012.
Subcortical motor circuit excitability during simple
and choice reaction time. Behavioral neuroscience,
126(3), pp.499–503.
McMillan, S., Nougier, V. & Byblow, W., 2004. Human
corticospinal excitability during a precued reaction
time paradigm. Experimental brain research, 156(1),
pp.80–7.
Müller, S. & Abernethy, B., 2012. Expert anticipatory skill
in striking sports: a review and a model. Research
quarterly for exercise and sport, 83(2), pp.175–87.
Nakamoto, H. & Mori, S., 2012. Experts in fast-ball sports
reduce anticipation timing cost by developing
inhibitory control. Brain and cognition, 80(1), pp.23–
32.
Regan, D., 1992. Visual judgements and misjudgements in
cricket, and the art of flight. Perception, 21(1), pp.91–
115.
Reynolds, C. & Ashby, P., 1999. Inhibition in the human
motor cortex is reduced just before a voluntary
contraction. Neurology, 53(4), pp.730–5.
Runigo, C. Le, Benguigui, N. & Bardy, B. G., 2005.
Perception-action coupling and expertise in
interceptive actions. Human movement science, 24(3),
pp.429–45.
Runigo, C. Le, Benguigui, N. & Bardy, B. G., 2010.
Visuo-motor delay, information-movement coupling,
and expertise in ball sports. Journal of sports sciences,
28(3), pp.327–37.
Soto, O., Valls-Solé, J. & Kumru, H., 2010. Paired-pulse
transcranial magnetic stimulation during preparation
for simple and choice reaction time tasks. Journal of
neurophysiology, pp.1392–1400.
Starr, A. et al., 1988. Enhancement of motor cortical
excitability in humans by non-invasive electrical
stimulation appears prior to voluntary movement.
Electroencephalography and clinical neurophysiology,
70(1), pp.26–32.
Valls-Solé, J. et al., 1999. Patterned ballistic movements
triggered by a startle in healthy humans. The Journal
of physiology, 516, pp.931–8.
Wolpert, D. M., Ghahramani, Z. & Jordan, M., 1995. An
internal model for sensorimotor integration. Science,
269(5232), pp.1880–1882.
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
12
