
Functional and Structural Similarity between Insect and Human
Hearts
Electrocardiography of Insect Hearts for Screening of New Cardioactive Drugs
Karel Sláma
1
and Radek Aulicky
2
1
Institute of Entomology, Czech Academy of Sciences, Drnovská 507, 16100 Praha 6, Czech Republic
2
Department of Pest Control of Stored Produccts, Crop Research Institute, Drnovská 507, 16100 Praha 6, Czech Republic
Keywords: Peristaltic Myocardial Contractions, Tubular Heart, Heartbeat Reversal, Myogenic Heartbeat, Pacemaker
Nodus, Neuromuscular Paralysis.
Abstract: The primordial formation of insect and human hearts is orchestrated by similar sets of genes. The rhythmi
city of purely myogenic, peristaltic myocardial contractions of insect heart is determined by a posterior
pace-maker nodus, which is analogous to sinoatrial or atrioventricular pacemaker nodi of the human heart.
Insects are very mobile animals; there are vigorous neuromuscular contractions and extracardiac pulsations
in haemocoelic pressure, which may seriously interfere with recordings of the heartbeat. This problem was
solved by using larvae of the waxmoth, whose neuromuscular functions were totally paralysed by
proteinaceous venom of the parasitic braconid wasp. The paralysed larvae survived motionless for 3 to 4
weeks, however, the regular myogenic pulsations of the heart and intestine, regulated by depolarisation
potentials of the myocardial or intestinal cells, remained fully preserved. The paralysed larvae of the
waxmoth are ideal object for cardiological research. By means of a touch-free, optoelectronic method we
found that the larval heart exhibited uninterrupted, forward-oriented (anterograde), peristaltic waves of
systolic myocardial contractions, propagated with a rate similar to that of the human heart (at 37°C).
Extensive screening of various cardioactive drugs revealed that the larval heartbeat, like the human
heartbeat, was sensitive to chronotropic action of digitoxine and the nitrates or cardiomoderating action of
verapamil.
1 INTRODUCTION
The recent availability of electronic recording
techniques (Sláma, 2003; 2010), have revealed
unexpec-ted similarities between the physiological
systems of insects and mammals. For example, the
autonomic, cholinergic neuroendocrine system
regulating insect respiration is structurally and
functionally analogous to the mammalian
parasympathetic nerve system (Sláma, 2008a; 2012).
The mapping of the human and insect (Drosophila)
genome have revealed that the primordial formation
and later functioning of insect and human hearts
were orchestrated by identical sets of genes (review
by Bodmer et al., (2005). And, as a matter of fact,
the pulsations of insect and human hearts are
regulated by similar, purely myogenic mechanisms
based on depolarisation potentials of myocardial
cells. In both cases the rhythmicity depends on a
special regulatory nodi (atrioventricular and
sinoatrial node of the human heart) (Hampton,
2003), or terminal regulatory node in the heart of
insects (Sláma, 2008a); (Sláma and Lukáš, 2011).
When genomic structures were still unknown,
comparative physiological studies were justified
only between the closely related groups of animals.
The mapping of the human and insect genomes,
however, has created a substantially new situation,
which is limited only by a serious lack of physio-
logical data. This is true for electrocardiographic
(ECG) records, which are mostly available only for
the human heart.
The human heart is a compact muscular organ,
which pumps blood into a closed vascular system of
arteries, capillaries and veins, under increased pres-
sure and at a constant temperature of 37°C. The
main function of this mammalian circulatory system
is linked with respiration; the circulating blood is
carrying oxygen from the lungs to distant organs and
prevents respiratory acidaemia by removing the me-
5
Sláma K. and Aulicky R..
Functional and Structural Similarity between Insect and Human Hearts - Electrocardiography of Insect Hearts for Screening of New Cardioactive Drugs.
DOI: 10.5220/0004615800050012
In Proceedings of the International Congress on Cardiovascular Technologies (CARDIOTECHNIX-2013), pages 5-12
ISBN: 978-989-8565-78-5
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

tabolically produced carbonic acid in the form of
carbon dioxide. Insects do not use a closed cir-
culatory system of arteries, veins and capillaries.
Instead of the lungs, they breathe through a seg-
mentally arranged system of spiracles and air-filled
tracheal tubes, which are ramified all over the body,
thus transporting aerial oxygen directly to tissue and
cells. The insect ‘‘blood’’ (haemolymph) circulates
between the three major body compartments (head,
thorax, abdomen), which are mutually inter-
connected and form an open body cavity, or
haemocoelic cavity (Jones, 1977); (Miller, 1997).
The dorsal vessel of adult insects consists of a
narrow elastic tube called the thoracic aorta and a
larger abdominal portion that is conventionally
called the insect heart in the strict sense. The
myocardium is segmentally prearranged, with
several pairs of usually incoming ostial valves,
perpendicular allatal muscles and pericardial
nutritive cells. In general, the insect heart is a tubular
organ, propagating waves of peristaltic contractions
in the forward direction (larvae), or alternatively in
both forward and backward direc-tions, which is
known as the heartbeat reversal. Recent
investigations show that, in comparison to the
human heart, the dorsal vessel of insects is a
relatively weak circulatory organ which is unable to
pump blood against any large barrier of mechanical
pressure (Sláma, 2000; 2012). Accordingly, the
insect heart is mostly used for mixing haemolymph
between the capital, thoracic and abdominal
compartments of the widely open body cavity. Due
to the limited, though very economic, pumping
ability of their heart, insects have evolved a number
of auxiliary circulatory adaptations such as the
accessory pulsatile organs of the appendages,
peristaltic pulsations of the intestine or strong extra-
cardiac pulsations in haemocoelic pressure (review
Sláma, 2008).
Insects and mammals are phylogenetically very
distant groups of animals (Prostomia and Deutero-
stomia) separated by millions of years of indepen-
dent evolutionary pathways. In spite of this, how-
ever, there exists well substantiated genetic evidence
that both insect (Drosophila) and human hearts share
some common morphogenetic principles (review by
Bodmer et al., (2005). Of particular interest in this
respect is a Tinman (Tin) gene containing a
transcriptional factor for the primordial heart in both
Drosophila and the human body (Ocorr et al.,
2007a); (Zeitouni et al., 2007). Comparisons
between the two phylogenetically distant circulatory
systems have been hampered for a long time by
superficially different anatomical and physiological
structures. Re-cently it has been found, however,
that both insect and human hearts are regulated by
similar, involun-tary and purely myogenic
mechanisms (Sláma and Lukáš, 2011). It also
appears that the rhythmicity of systolic cardiac
contractions in insects depends on a special
pacemaker nodus (Terminal regulatory no-dus)
(Sláma, 2006; 2012), which has a similar phy-
siological role to the atrioventricular, sinoatrial, or
Hiss bundle pacemaker nodi of the human heart
(Hampton, 2003). In addition to segmental, peristal-
tically propagated systolic contractions, certain
insect species have evolved a compact, conical
ventricle in the heart, characterised by a human-like
atrium and synchronic, not peristaltic, mode of
cardiac contractions (Sláma, 2010). Similarities be-
tween insect and human hearts prompted me to
investigate the possibility of similar responses with
respect to medicinal cardioactive drugs. The assays
were facilitated by the development of noninvasive,
touch-free, electrocardiographic methods for insects
(Sláma, 2003; 2006; 2012); (Sláma and Lukáš,
2011).
It appeared that the common cardioactive drugs
(noradrenaline, digitoxine, nitrates, Ca2+ ion
blockers, indeed produced heartbeat responses in
insects similar to those found in the human heart.
Encouraged by the results obtained in Drosophila
(Occor et al., 2007b); (Fink et al., 2009); (Sláma,
2010), I also looked at the hearts of some other flies.
Of particular interest was a family of hoverflies
(Syrphidae) including the presence of several real
champions in sustained flight. One species,
Episyrphus balteatus, revealed a quite uncommon,
anatomically and functionally human-like heart,
with a compact ventricle pumping the insect
‘‘blood’’ into an artery-like aorta (Sláma, 2013).
Here I describe new ECG data for an insect heart
and try to find possible analogies with the known
facts in human cardiology.
2 RESULTS AND DISCUSSION
2.1 Electrocardiography of Pupal
Hearts
The depolarization and repolarization electrical
potentials created by intensive contractions of the
compact human myocardium can be successfuly
recorded by external electrodes located at different
parts of the body. This represents the common prin-
ciple of ECG recordings in medicine (Hampton,
2003). The contracting insect myocardial cells exhi-
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
6
bit similar depolarization and repolarization poten-
tials. However, these potentials are smaller and less
convenient for recording by external electrodes,
because: 1. The myocardium of an insect tubular
heart is divided into metameric segmental compart-
ments, which contract sequentially in the forward
(anterograde heartbeat) or backward (retrograde
heartbeat) directed waves of peristaltic contractions;
2. The rhythmicity of cardiac pulsations is deter-
mined by a pacemaker nodus located in posterior
segments of the heart; 3. Each peristaltic wave of
myocardial contractions can occasionally start
before the previous wave arrived to its final
destination (more peristaltic waves running at the
same time). These data show that, in contrast to the
human heart, the heart of insects creates relatively
small and dispersed depoarization potentials, which
are difficult to be directly recorded.
We are reasonably thinking, however, that due to
recent progress in electronic recording techniques, it
will be soon possible to monitor electrical depolari-
sation potentials of insect myocardial segments, just
like in ECG of the human heart. Recently we have
developed several ECG methods, which enable
accurate monitoring of myocardial contractions in
the insect heart. The methods cannot record the
depolarisation potentials directly, but reveal
precisely the myocardial contractions in one or
multiple segments of the body. So far, we have
obtained the best results with two, previously
described ECG methods suitable for insect heart
(Sláma, 2003); (Sláma and Miller, 2001), i. e.
thermocardiographic and optocardiographic
methods. In principle, the thermocardiographic
method is based on the use of miniature thermistor
sensors positioned externally above the pericardial
sinus of the heart. The sensors are gently warmed to
create a temperature gradient around their bodies.
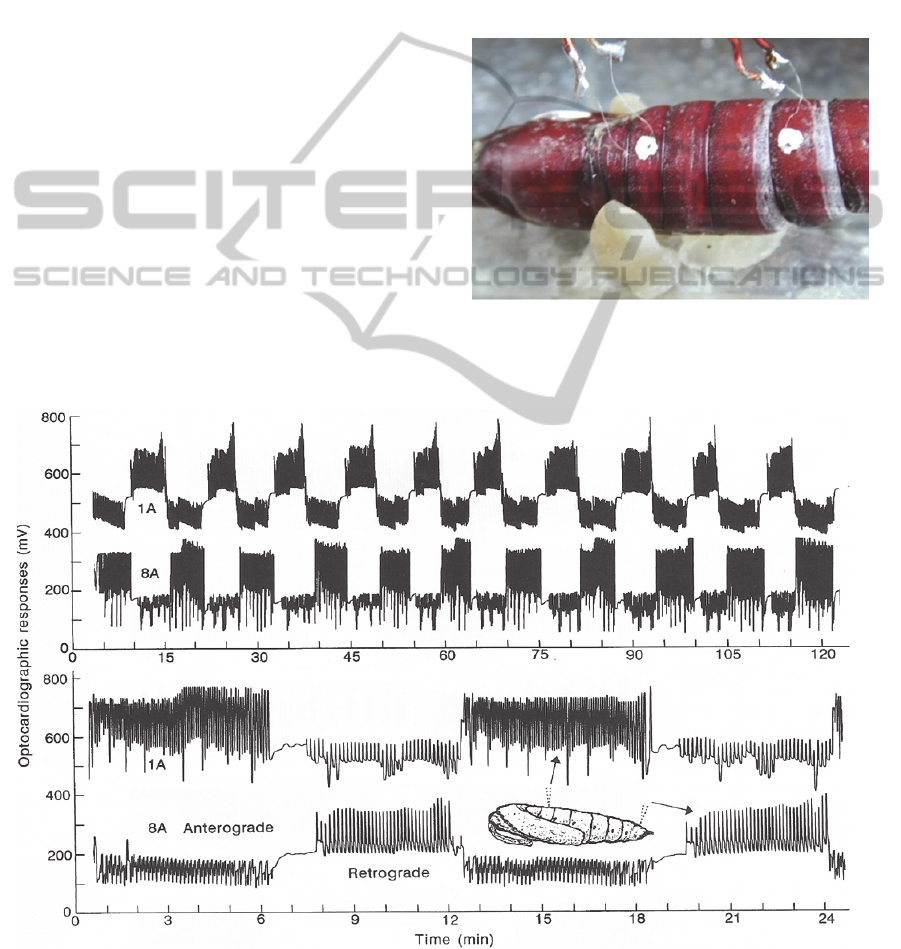
Figure 1: Miniature thermographic sensors positioned
externally over the heart of a diapausing pupa of Manduca
sexta (from Sláma, 2003).
Figure 2: Heartbeat reversal recorded by two thermographic sensors from the first (1A) and eight (8A) body segments of
diapausing pupa of Manduca sexta. Lower portion shows the detail with expanded time scale (From Sláma, 2003).
FunctionalandStructuralSimilaritybetweenInsectandHumanHearts-ElectrocardiographyofInsectHeartsforScreening
ofNewCardioactiveDrugs
7

Figure 3: Effect of digitoxine injection (arrow; 20 mg/kg of body mass) on pupal heartbeat in Manduca sexta.
The subintegumental movements of haemolymph
caused by pulsations of the heart disturb the
established temperature gradients around the sen-
sors, which are converted into the corresponding
electrical sisgnals that are finally recorded by the
recording devices (see Figure 1 and 2).
The recording of insect heartbeat is often frus-
trated by movements of somatic muscles and by
special extracardiac pulsations in haemocoelic pres-
sure, used for inspiration and expiration of air
through spiracles. Suitable developmental stages for
recording the heartbeat of insects are immobile
pupae (Figure 1). The records in Figure 2 show that
the peristaltically propagated waves of myocardial
contractions move alternatively forwards and back-
wards. The rhythmicity is determined by a pace-
maker nodus located in posterior region of the heart.
After sectioning in the middle of the heart, posterior
section preserved rhythmicity and periodicity of
heartbeat reversal, whereas anterior section lacking
the pacemaker nodus exhibited only some slow,
indifferent contractions similar to human heart de-
prived from the ventricular nodus (Sláma, 2006).
The forward oriented, anterograde heartbeat, which
pumps abdominal haemolymph into the head,
represents the most important circulatory function.
There are numerous insect larvae which show only
this unidirectional, forward oriented anterograde
cardiac pulsation. In Figure 2, the thermographic
sensors located at the base of pupal abdomen reveal
increased amplitude of haemolymph circulation
during anterograde heartbeat. During the reciprocal,
retrograde heartbeat the relationships are reversed.
Diapausing pupae of Manduca sexta (Figure 1) are
very convenient for recording of insect heartbeat.
They can be stored in refrigerator for several months
before use. Figure 3 shows an example of chrono-
tropic effect of digitoxine injection on pupal heart-
beat of Manduca. Using this model system, we
tested a number of cardio-stimulatory or inhibitory
pharmacological preparations and found similar
structure-activity relationships known from the hu-
man medicine (Sláma, 2008b).
2.2 Optocardiography of Larval and
Pupal Hearts
Thermographic sensors need to be firmly attached to
integumental surface over the pericardial region.
This condition can be satisfied in immobile pupae,
but not in the mobile larvae with smooth and elastic
cuticle. These obstacles were restrained by
development of absolutely touch free, optoelectronic
techniques. The device shown in Figure 4 uses four
independent channels of red pulselight, focused to a
small (0.3 mm
2
) area over the heart through optic
fibers and lenses from the distance of 7 mm. The
beam of constant, stabilised pulse-light is applied to
the measured epidermal area by one optic fiber.
Changes in optical density, which are caused by
contractions of the heart, are dispatched to photo-
multipliers of the device by incoming optic fibers.
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
8

After multiplication and decoding, the heartbeat
pulsations are converted into output voltage and are
recorded. Figure 4 shows immobile pupa of Zop-
hobas atratus during touch-free recording of heart-
beat. Optocardiographic recordings could be exe-
cuted in all stages with reasonably transparent cuti-
cle and in specimens which do not move for a short
time. The dorsal vessel of insects (insect heart) is
located just under the integumental cover. Many
insects are resting immobile for shorter or longer
periods of time. Their heartbeat can be easily recor-
ded by optocardiographic methods, simply by focu-
sing the pulse-light beam over the pericardial region,
such as in case of M. sexta caterpillar during feding
(see Figure 5).
Figure 4: Four-channel optoelectronic device used for
pulse-light optocardiographic recording of heartbeat.
Figure 5: Caterpillar of M.sexta during the pulse- light,
optoelectronic recording of heartbeat by Sláma (2003).
2.3 Heartbeat during Neuromuscular
Paralysis
Larvae of the greater waxmoth (G. mellonella) are
perhaps the best experimental model of insects.
They are adjusted to live in 37°C of the bee hives. In
the laboratory, they can be reproduced in large
numbers at any time of the year. The larvae exhibit
unidirectional, purely anterograde heartbeat with the
rates comparable to that of the human heart (at
37°C). The problem is that these larvae are extre-
mely mobile and have rather fast developmental
rate. Under these conditions, recording of heartbeat
is very difficult. A great progress in this respect has
been achieved few years ago when Sláma and Lukáš
(2011) found that larvae, which were subjected to
neuromuscular paralysis induced by venom of the
parasitic braconid wasp, exhibited apparently normal
heartbeat. The profound neuromuscular paralysis,
which could last for several weeks, affected all so-
matic muscles innervated through neuromuscular
transmission. The peristaltic contractions of the heart
and intestine, however, which were regulated by de-
polarisation potentials of the myocardium, remained
unaffected and fully functional. The heartbeat
patterns of these motionless, paralysed larvae can be
easily monitored by all types of the recording me-
thods. Electrocardiographic investigations on these
larvae revealed the autonomic (brain independent)
nature of heartbeat regulation. Further advantage of
Galleria depends on the prolonged survival when
deprived of the brain hormone source by ligatures
made behind the head. The ligatured larvae with
arrested development (Figure 6 (A)) can be stored
for many weeks for later use. In combination with
additional paralysis by the braconid venom (Figure 6
(B)), these motionless larvae represent the best
experimental material for routine screenings of va-
rious cardio-active materials on insects. Due to total
absence of the somatic movements, it was possible
to use the pralysed larvae for prolonged optocar-
diographic recordings of the heartbeat. The use of
multiple optocardiographic sensors (see Figure 7),
enabled determination of the propagation velocity of
individual systolic contraction waves.
Earlier recordings (Sláma and Lukáš, 2011)
revealed more or less constant cardiac pulsations in
the paralysed larvae, characterised by 20–25 systolic
contractions per minute. The contractions were peri-
staltically propagated in the forward (anterograde)
direction with a more or less constant speed of 10
mm per second (23–25°C). Sectioning performed in
the middle of the heart (4th abdominal segment)
seriously impaired the pacemaker rhythmicity and
slowed down the rate of heartbeat in the anterior
sections. By contrast, the functions of the posterior
compartment of the disconnected heart remained
unaffected.
FunctionalandStructuralSimilaritybetweenInsectandHumanHearts-ElectrocardiographyofInsectHeartsforScreening
ofNewCardioactiveDrugs
9
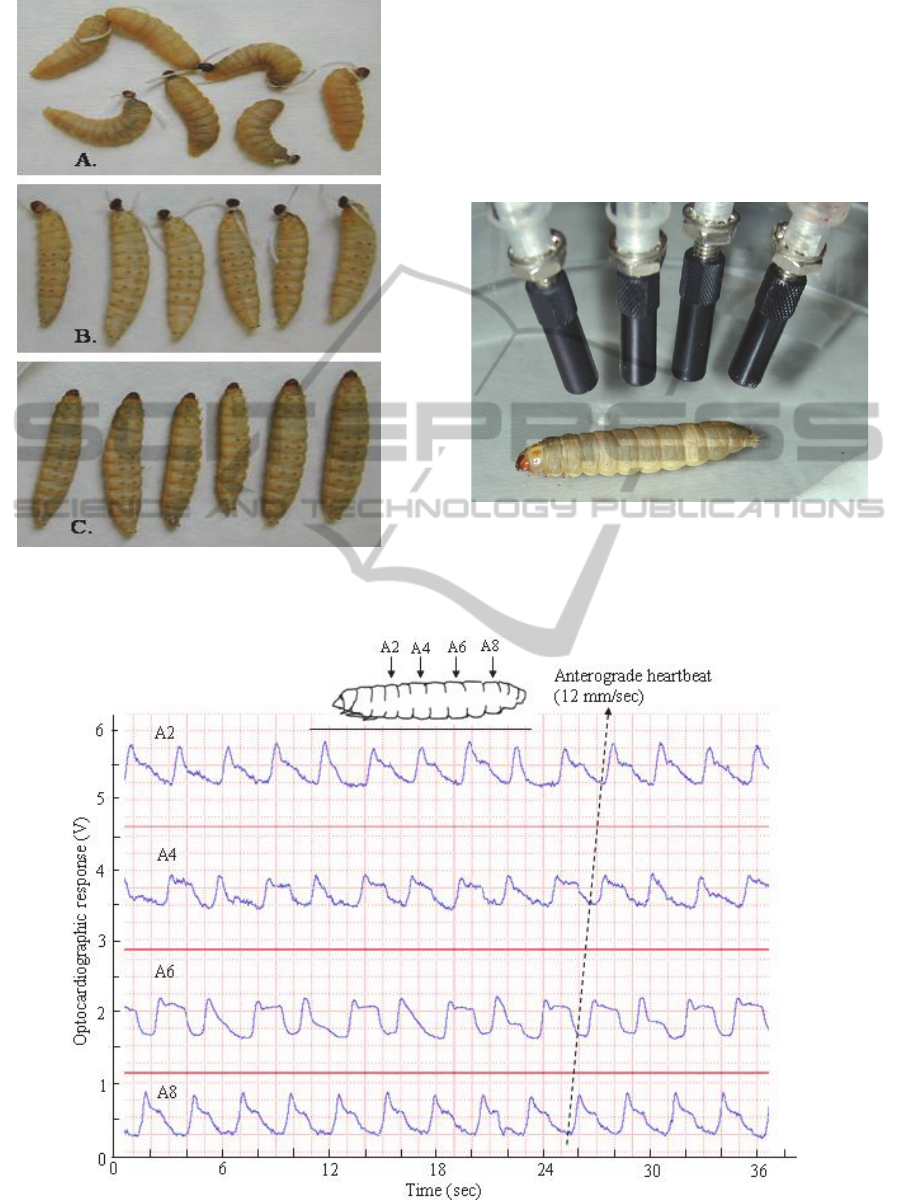
Figure 6: The fully grown larvae of Galleria, which have
been ligatured behind the head (A), ligatured and injected
with braconid venom (B) or injected without ligaturing.
An example of simultaneous, 4 channel
optocardio-graphic, ECG-like record with the
paralysed larva (as shown in Figure 7), can be found
in Figure 8. The multichannel records of this type
reveal the details of systolic contractions of the
heart. In addition to the rate of heartbeat, these
records reveal the propagation velocity of individual,
systolic contraction waves.
Figure 7: Paralysed larva of Galleria during multiple
optocardiographic recordings.
Figure 8: Example of simultaneous optocardiographic record of heartbeat in paralysed larva of Gallera, obtained by means
of the multichannel setup shown in Figure 7 (from Sláma, 2011).
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
10
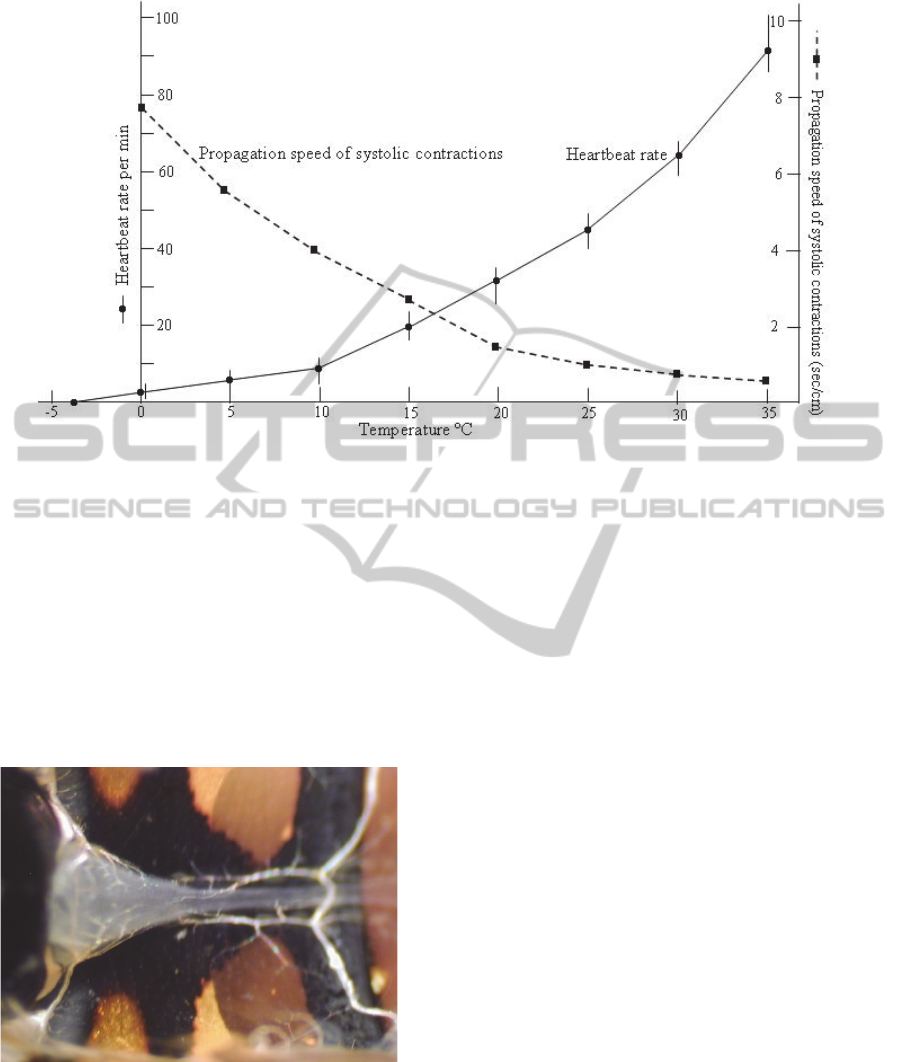
Figure 9: Effect of different temperatures on heartbeat rate and velocity of peristaltic myocardial contractions in the
paralysed larva of Galleria (from Sláma, 2012a).
Figure 9 gives detailed acount of the effect of
different temperatures on the rate of heartbeat in the
paralysed larva of Galleria. The larvae are paralysed
by purified extract prepared from the venom glands
of the tiny braconid wasp, Habrobracon hebetor
(10
glands per ml of Ringer, 20 µl injection).The results
show that the rate of heartbeat pulsation is between
80 and 90 pulses per min and the velocity of
peristaltic myocardial contractions is less than one
second, at 35°C.
Figure 10: Compact, human-like heart ventricle seen from
the ventral side of abdomen in an adult hover fly,
Episyrphus balteatus. The ventricle is stretched within the
body cavitly by lateral ligaments (left), with a narrow
posterior heart (right) (From Sláma, 2013).
The compact ventricle found in the hoverfly,
shown in Figure 10, represents a new and enigmatic
feature in comparative animal physiology. The
heartbeat rate shows real animal recod (frequency
over 10 Hz) and, what is most important, the
mechanism is not peristaltic, but synchronic, like in
the human heart (the whole ventricle composed of
several metameric segments contracts unisono). The
compact muscular ventricle and synchronic
contraction mechanism are apparently the
physiological adaptations to increasing demands for
the forward oriented, anterograde pumping of
haemolymph to extensively developed, thoracic
flight musculature.
3 CONCLUSIONS
We are reasonably thinking that due to the described
functional similarities between insect and human
hearts, the new cardioactive or inhibitory substances
can be tested on the hearts of insects. The biassays,
performed by the described, touch-free cardiogical
methods on the paralysed larvae of Galleria, can
potentially serve as a convenient and inexpensive
way how to avoid the use of large animals for
biological testing of cardiologically important
chemicals.
FunctionalandStructuralSimilaritybetweenInsectandHumanHearts-ElectrocardiographyofInsectHeartsforScreening
ofNewCardioactiveDrugs
11

ACKNOWLEDGEMENTS
We acknowledge with thanks financial support from
NAZV granting agency, Czech republic, for roject
No. QJI310057 to R.A.
REFERENCES
Bodmer, R., Wessells, R. J., Johnson, E. C., Dowse, H. B.,
2005. Heart development and function. In:Gilbert, L.
I., Iatrou, K., Gill, S. (Eds.), Comprehensive
Molecular Insect Science, Vol. 2, 199–250.
Fink, M., Callol-Massot, C., Chu, A., Ruiz-Lozano, P.,
Belmonte, J. C. I., 2009. A new method for detection
and quantification of heartbeat parameters in
Drosophila, zebrafish and embryonic mouse hearts.
Bio Techniques 46, 101-113.
Hampton, J. R., 2003. The ECG in Practice, Fourth ed.
Elsevier Limited, Oxford.
Jones, J. C., 1977. The circulatory system of insects.
Charles, C.Thomas. (Ed.) Springfield, Ill.
Miller, T. A., 1997. Control of circulation in insects. Gen.
Pharmacol. 29, 23–38.
Ocorr, K., Perrin, L., Lim, H.-Y., Qian, L., Wu, X.,
Bodmer, R., 2007a. Genetic control of heart function
and aging in Drosophila. Trends in Cardiovascular
Medicine 17, 177–182.
Ocorr, K., Reeves, N. L., Wessels, R. J., Fink, M., Chen,
H. S., 2007b. KCNQ potassium channels mutations
cause cardiac arrhythmias in Drosophila that mimic
the effectřs of aging. Proc. Natl. Acad. Sci. USA, 104,
3943-3948.
Sláma, K., 2000. Extracardiac versus cardiac haemocoelic
pulsations in pupae of the mealworm (Tenebrio
molitor L.). J. Insect Physiol. 46, 977–992.
Sláma, K., 2003. Mechanical aspects of heartbeat reversal
in pupae of Manduca sexta J. Insect Physiol. 49, 645–
657.
Sláma, K., 2006. Heartbeat reversal after sectioning of the
dorsal vessel and removal of the brain in diapausing
pupae of Manduca sexta, Lepidoptera: Sphingidae).
Eur. J. Entomol. 103, 17–26.
Sláma, K., 2008a. Extracardiac haemocoelic pulsations
and the autonomic neuroendocrine system
(coelopulse) of terrestrial insects. Terrestrial
Arthropod Reviews 1, 39–80.
Sláma, K., 2008b. Method of testing cardioactive
preparations on insects. Czech Patent Application
PV 2008–671.
Sláma, K., 2010. Physiology of heartbeat reversal in adult
Drosophila melanogaster (Diptera: Droso-philidae).
Eur. J. Entomol. 107, 13–31.
Sláma, K., 2012. Neuromuscular paralysis induced in
insect larvae by the proteinic venom of a parasitic
wasp. In: Paralysis: Causes Classification and
Treatment , Nova Science Publishers Inc., New York,
pp. 1–24.
Sláma, K., 2013. A new look at the comparative
physiology of insect and human hearts. J. Insect
Physiol. 58, 1072-1081.
Sláma, K., Lukáš, J., 2011. Myogenic nature of insect
heartbeat revealed by neuromuscular paralysis caused
by the sting of a braconid wasp. J. Insect Physiol. 57,
251–259.
Sláma, K., Miller, T. A., 2001. Physiology of heartbeat
reversal in diapausing pupae of the tobacco hornworm,
Manduca sexta (Lepidoptera: Sphin-gidae). Eur. J.
Entomol. 98, 415–431.
Zeitouni, B., Senatore, S., Severac, D., Aknin, C.,
Semeriva, M., Perrin, L., 2007. Signalling pathways
involved in adult heart formation revealed by gene
expression profiling in Drosophila. PLoS Genetics 3,
1907–1921.
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
12
