
Impact of Pericardial Effusion on Cardiac Mechanics in Patients
with Dilated Cardiomyopathy
Francesco Scardulla
1
, Antonino Rinaudo
1
, Cesare Scardulla
2
and Salvatore Pasta
3
1
Dipartimento di Ingegneria Chimica, Gestionale, Informatica e Meccanica, Universita' di Palermo,
Viale delle Scienze Ed. 8, 90128 Palermo, Italy
2
Mediterranean Institute for Transplantation and Advanced Specialized Therapies (ISMETT),
Via Tricomi n.1, 90127, Palermo, Italy
3
Fondazione RiMED, Via Bandiera n.11, 90133, Palermo, Italy
Keywords: Finite Element Analysis, Cardiac Mechanics, Cardiomyopathy, Pericardial Effusion.
Abstract: Dilated cardiomyopathy (CDM) is a degenerative disease of the myocardium accompanied by left
ventricular (LV) remodeling, resulting in an impaired pump performance. Differently, pericardial effusion
(PE) is a liquid accumulation in the pericardial cavity, which may inhibit blood filling of heart chambers.
Clinical evidence show that PE may improve pump performance in patients with CDM. Therefore, this
study aims to assess wall stress and global function of patients with CDM, PE as compared to healthy
patient. These findings suggests that CDM has an important implication in the mechanical changes of LV
and right ventricle by increasing wall stress and reducing pump function. Conversely, PE determines
lowering myocardial fiber stress and improves global function as compared to those of CDM.
1 INTRODUCTION
Dilated cardiomyopathy (CDM) is a degenerative
disease of the myocardial tissue accompanied by left
ventricular (LV) remodeling (Nakayama et al.,
1987). The histologic characteristics of CDM
include hypertrophy of myofibers, myofibrillar lysis,
nuclear changes and vacuolization of myocardial
fibers and interstitial fibrosis of the myocardium
(Hayashida et al., 1990). LV remodeling is a
multistep process that involves acute dilation of the
infarcted area, increase of LV volume, lengthening
of the LV perimeter, and decrease of LV curvature.
Natural history studies show that progressive LV
remodeling is directly related to future deterioration
of LV performance and a poor clinical course (Cohn
et al., 2000) (Swynghedauw, 1999).
Pericardial effusion (PE) is a pathological
accumulation of fluid within the pericardial space
(Mirhosseini et al., 2013). Usually, such disease do
not influence clinical decision-making as long as the
PE is not considered haemodynamically
compromising cardiac functionality (Frohlich et al.,
2013). PE fluid accumulation can be attributed to an
underlying systemic or local inflammatory process
such as cancer or myo-/pericarditis or might occur
after surgery or can be secondary to congestive,
severe heart failure. However, the mechanism of PE
development and its prognostic value in heart
failure remain elusive. A persistent PE at
echocardiographic follow-up was associated with
unfavourable outcome when compared with patients
with resolved PE. Indeed, patients with PE exhibit
worse right ventricular (RV) function, larger right
atrial dimensions, more pronounced tricuspid
regurgitation as well as a higher prevalence of
pulmonary hypertension. A recent study shows that
patients with PE have significantly elevated RA
filling pressures and an increased mean arterial
pulmonary pressure, whereas the left ventricular
filling pressure and wedge pressure did not differ
between PE and control group (Frohlich et al.,
2013).
Mathematical modeling of the cardiovascular
system using the finite element (FE) approach is an
useful too to estimate the cardiac mechanics and
wall stress, likely inhibiting CDM and PE. A few FE
modeling studies of the LV have validated stress
calculations by showing good agreement with
myocardial strain measured with implanted markers
(McCulloch et al., 1992); (Omens et al., 1993);
(Vetter and McCulloch, 2000). Guccione et al. have
639
Scardulla F., Rinaudo A., Scardulla C. and Pasta S..
Impact of Pericardial Effusion on Cardiac Mechanics in Patients with Dilated Cardiomyopathy.
DOI: 10.5220/0004615906390644
In Proceedings of the 3rd International Conference on Simulation and Modeling Methodologies, Technologies and Applications (BIOMED-2013), pages
639-644
ISBN: 978-989-8565-69-3
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

successfully modeled end-isovolumic systole in an
ovine model of myocardial infarction and
determined material parameters that reproduced
circumferential stretching (as measured with 2D
tagged MRI) in the infarcted border zone (Guccione
et al., 2001).
The key role of wall stress in the progression of
LV remodeling was studied in DCM (Quarterman et
al., 2002); (Ratcliffe et al., 1998); (Zhong et al.,
2009). An increase in wall stress is known to reduce
myocardial fiber shortening, and increases in LV
wall stress have been reported in DCM. LV wall
stress is in part determined by the local curvature of
the ventricular wall (i.e., decreased curvature will
increase wall stress). In addition to increasing LV
size, CDM can alter myocardial properties and
normal LV shape curvature. The border zone will
have a higher stress, which makes it more
susceptible to ischemia and infarction and may
accelerate the remodeling process.
Therefore, the purpose of present study was to
assess the key role of wall stress and global function
of patients with CDM, PE. Cardiac mechanics was
thus compared to that of healthy patients with
normal wall thickness. We also tested the hypothesis
that pump function and wall stress in CDM can be
positively affected by the presence of a PE liquid.
2 MATERIALS AND METHODS
2.1 Imaging Procedure
We retrospectively identified patients with CDM
and PE who underwent magnetic resonance imaging
(MRI) from radiologic records of Mediterranean
Institute for Transplantation and Advanced
Specialized Therapies (ISMETT) and Ospedale
Riuniti Trieste. Patients underwent MRI as part of
their care, and not for the purpose of our study.
A series of long- and short-axis images of the
heart were obtained performing MR imaging
synchronized to the R wave of the electrocardiogram
signal. Short -axis slices were taken sequentially
every 6 mm until complete scanning of heart
chambers.
2.2 Heart Reconstruction
Endocardial and epicardial MRI surfaces of LV and
RV were segmented by contour lines using the
vascular modeling toolkit VMTK
(http://www.vmtk.org). Specifically, LV and RV
geometries were reconstructed at end-diastole (ED)
and end-systole (ES), which are defined as the
images with the maximum and minimum cross-
sectional area, respectively. Patients with PE
required also segmentation of the outer pericardial
layer. After segmentation process, endocardial and
epicardial surfaces of LV and RV were obtained by
loft protrusion of segmented contour lines.
2.3 FE Model
The space between the endocardial and epicardial
surfaces was meshed with 8-node brick elements to
generate a volumetric mesh in ABAQUS FE code.
Cardiac myofiber angles at the epicardium and
endocardium were assigned to be -60 degrees and 60
degrees, respectively (counterclockwise positive
when viewed from the epicardium).
Nearly incompressible, transversely isotropic,
hyperelastic constitutive laws for passive and active
myocardium was implemented in ABAQUS/Explicit
using a VUMAT subroutine (Ratcliffe et al., 1998).
Myocardial material parameters were estimated
comparing MRI measured and computationally
derived LV and RV volumes at ED and ES,
respectively. Manual iteration was used rather than
formal optimization. Similarly, PE was modeled as
isotropic, hyperelastic material which mechanical
properties were empirically found to match ED and
ES volumes of PE.
The basal node of LV were constrained along
long axis direction. The endocardial wall was loaded
at ED and ES pressure occurring at LV and RV. For
LV, ED pressure (P
ED
) was 100 mmHg while ES
pressure (P
ES
) was 25 mmHg. For RV, P
ED
was 15
mmHg while P
ES
was 30 mmHg.
2.4 Pressure-volume Relationships
and Stroke Volume
Chamber ED and ES volume (V
ED
and V
ES
)
solutions were used with the corresponding P
ED
and
P
ES
to plot the ED and ES pressure-volume
relationships (ESPVR and EDPVR, respectively),
which were then fit to appropriate polynomial
equations. The following linear equation was used to
estimate the ESPVR:
P
E
V
V
(1)
where E
ES
is the end-systolic elastance and V
0
is the
volume intercept of the ESPVR, each determined by
linear regression of the data.
The polynomial equation used to estimate the
EDPVR was as follows:
P
E
,
E
,
V
E
,
V
(2)
SIMULTECH2013-3rdInternationalConferenceonSimulationandModelingMethodologies,Technologiesand
Applications
640
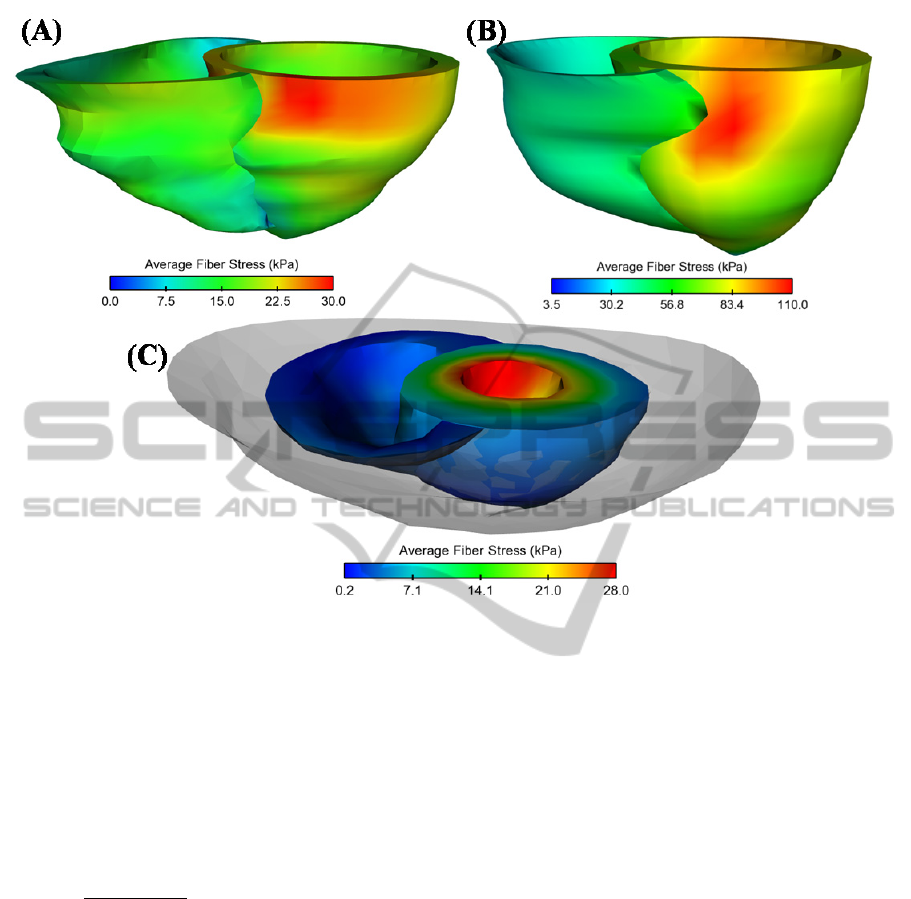
Figure 1: Representative map of average wall stress for (A) healthy patient, (B) CDM and (C) PE (solid grey indicates
liquid volume); models are not at same scale.
where E
0,ED
, E
1,ED
and E
2,ED
represent stiffness of the
LV diastolic compliance, again determined by linear
regression.
To determine global changes to pump function,
the stroke volume (SV)/P
ED
and SV/V
ED
relationships were calculated and plotted, assuming
that arterial elastance (E
A
) was constant. SV was
calculated according to the following equation:
1
/
(3)
2.5 ED and ES Fiber Stress
For each simulation, stress in the local muscle fiber
direction was computed throughout the LV and RV
walls at end-diastole and end-systole of the pressure-
volume load. Thus, we evaluated the
average fiber stress has the mean value between the
longitudinal fiber stress and the cross-fiber stress.
3 RESULTS
Figure 1 shows representative distribution of
average fiber stress for patients with PE and CDM as
compared to the healthy patient. It can be observed
that ES LV stress is higher than that of RV, and this
occurs in the lateral side of LV chamber.
Differently, the patient with PE show higher ES wall
stress at the endocardial surface a cause of the liquid
constraining LV wall motion.
Maximum values of ES average fiber stress was
found markedly higher for patients with CDM
(105.5 kPa, n=3) compared to that of healthy patient
(20.9 kPa, n=2) and PE patient (42.9 kPa, n=2) as
shown by Figure 2. For both healthy and CDM
cases, maximum value of ED average fiber stress
was found drastically lower that those exhibited at
end-systolic phase (i.e., 13.5 kPa for healthy and
12.2 kPa for CDM). In contrast, ES fiber stress of
PE cases decreased up to 19.1 kPa.
For LV, CDM caused a leftward shift of both
EDVPR and ESPVR whereas PE induced a
rightward shift of these relationships as shown by
Fig. 3. Similar results are shown by RV. Starling
curve for CDM lies on the left compared to that of
both PE and healthy patients. Indeed, stroke volume
(Starling law) was reduced in CDM because the
decrease in diastolic compliance was not sufficiently
ImpactofPericardialEffusiononCardiacMechanicsinPatientswithDilatedCardiomyopathy
641
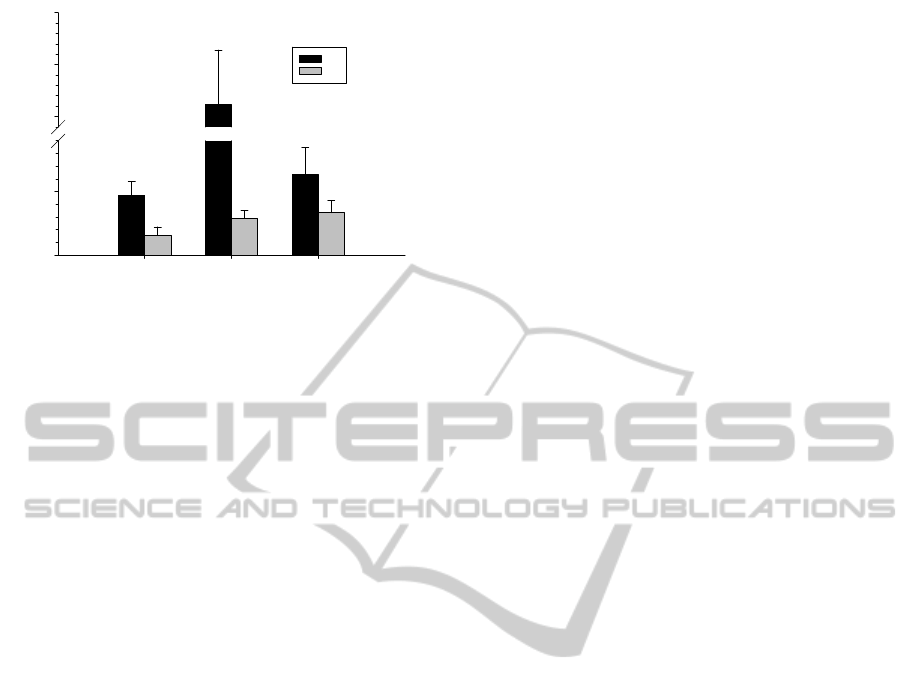
Figure 2: Comparison of maximum average wall stress
among patients with CDM and PE. Control, healthy
patient is also shown. Data are mean±SEM.
compensated by the improvements in end-systolic
elastance.
4 DISCUSSION
The present research demonstrates clearly that CDM
and PE alter differently both wall stress and cardiac
function when compared to healthy subject. Indeed,
the most striking finding is that the patient with PE
exhibits lower myocardial fiber stress and better
global function than those of the patient with CDM.
Therefore, both CMD and PE have important
implications in the mechanical changes of both LV
and RV chambers.
There are few studies on the wall stress and
cardiac function in CDM. Among these, Zhog et al.
investigated LV remodeling in ischemic CDM using
FE modeling (Zhong et al., 2009). They suggested
that LV remodeling in ischemic CDM is a multistep
process, which determines loss of contractile
function followed by acute dilatation of the
infarction area, increase of LV volume, lengthening
of the LV perimeter, and blunting of the normal
curvature. Wall stress were found increased in each
region of LV wall and has been shown to be a
measure of the afterload following infarction. These
findings are in agree with our distribution of wall
stress in CDM. Nevertheless, we found that ES wall
stress are 72% higher than that of healthy and PE
subjects, suggesting adverse clinical outcome for
this cardiac disease.
FE modeling has been widely used to study
cardiac diseases, and this has led to an improved
integrative understanding of the heart system. For
instance, Wenk et al. evinced that residual stress
produced by ventricular volume reduction surgery
has a little effect on the LV function and cardiac
mechanics (Wenk et al., 2010). Another study
suggests that surgical anterior ventricular restoration
reduces myofiber stress in the akinetic infarct at the
expense of a reduction in the Starling relationship
(Jhun et al., 2010). FE analysis also demonstrated
that aneurysm implication decreases fiber stress
without depressing stroke volume (Guccione et al.,
2001); (Walker et al., 2005). Recently, Carrick et al.
highlighted that Coapsys procedure decreases
myofiber stress at ED and ES, and that the
improvement in myofiber stress may contribute to
the long-term effect of Coapsys on LV remodeling
(Carrick et al., 2012).
In tissue engineering approach, FE simulation
indicated that the addition of non-contractile
material to a damaged LV wall has important effects
on cardiac mechanics, with potentially beneficial
reduction of elevated myofiber stresses, as well as
confounding changes to clinical left ventricular
metrics (Wall et al., 2006). This study therefore
supports our hypothesis that that pump function and
wall stress in CDM may be improved by
surrounding the epicardial layer with liquid as it
occurs in PE. This is also confirmed by clinical
evidence in patients with liquid accumulation for
which the global pressure-volume relationships is
shift further to the left as suggested by our
computational model.
6 MODEL LIMITATIONS
Although this model captures many aspects of LV
and RV mechanics in both CDM and PE, limitations
still exist. One significant limitation is that wall
thickness between patients with CDM and PE were
different.
This likely influences the wall stress which is
given by the ratio of the pressure exerted on the LV
endocardio on wall thickness. Indeed, patient with
PE had a LV wall thickness of 8.5 mm which is
higher than that of CDM (i.e., 5.7 mm). Future study
needs patient comparison at matched values of LV
wall thickness.
Other limits include calculation of regional
myocardial material properties as well as restricted
number of patients. In spite of this limitations, these
findings provide relevant insight on the cardiac
mechanics of patients with CDM and PE.
Healthy CDM PE
Max Average Fiber Stress (kPa)
0
50
200
250
300
ES
ED
(n=3)
(n=2) (n=2)
SIMULTECH2013-3rdInternationalConferenceonSimulationandModelingMethodologies,Technologiesand
Applications
642
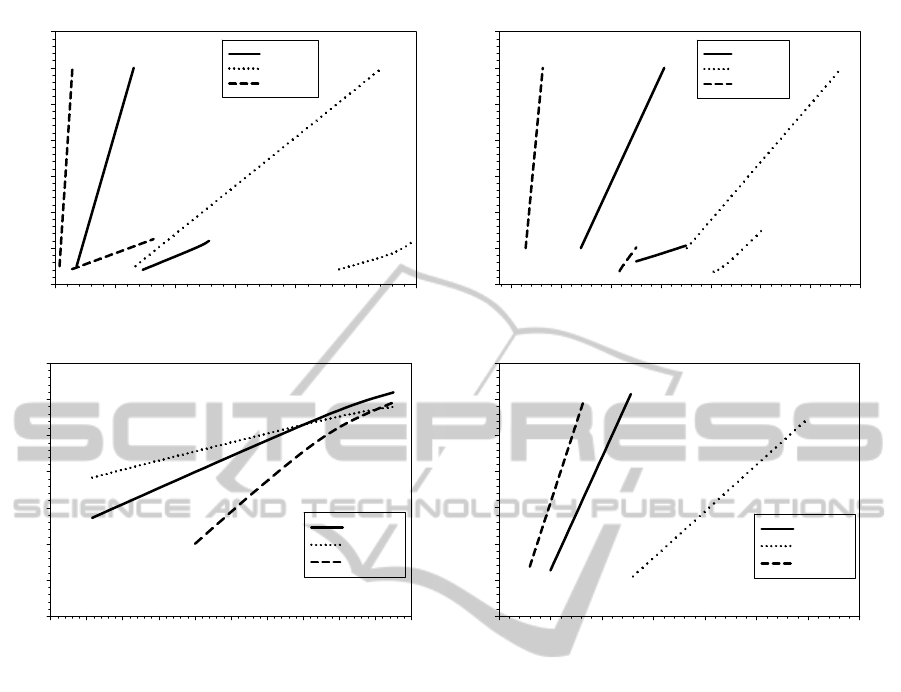
Figure 3: Representative cardiac function for a patient of each group: (A) LV pressure-volume relationships, (B) RV
pressure-volume relationships, (C) LV stroke volume on ED pressure and (D) LV stroke volume on ED volume.
7 CONCLUSIONS
This study suggests that CDM and PE conversely
alter both wall stress distribution and global cardiac
function. The reduction in the myofiber stress caused
by liquid accumulation on the pericardial layer may
contribute to the long-term clinical outcome of
patient with PE.
ACKNOWLEDGEMENTS
The authors thank Mr. Armando Pasta of ISMETT
for his technical assistance with image acquisition.
This study was funded in part by a grant from
Fondazione RiMED provided to Dr. Pasta.
REFERENCES
Carrick, R., Ge, L., Lee, L. C., Zhang, Z., Mishra, R.,
Axel, L., Guccione, J. M., Grossi, E. A., Ratcliffe, M.
B., 2012. Patient-specific finite element-based analysis
of ventricular myofiber stress after Coapsys:
importance of residual stress. Ann Thorac Surg
93:1964-1971.
Cohn, J. N., Ferrari, R., Sharpe, N., 2000. Cardiac
remodeling--concepts and clinical implications: a
consensus paper from an international forum on
cardiac remodeling. Behalf of an International Forum
on Cardiac Remodeling. J Am Coll Cardiol 35:569-
582.
Frohlich, G. M., Keller, P., Schmid, F., Wolfrum, M.,
Osranek, M., Falk, C., Noll, G., Enseleit, F.,
Reinthaler, M., Meier, P., Luscher, T. F., Ruschitzka,
F., Tanner, F. C., 2013. Haemodynamically irrelevant
pericardial effusion is associated with increased
mortality in patients with chronic heart failure. Eur
Heart J
Guccione, J. M., Moonly, S. M., Moustakidis, P., Costa,
LV ED Volume (ml)
0 50 100 150 200 250 300 350
Stroke Volume (ml)
10
20
30
40
50
60
70
80
Healthy
CDM
PE
LV ED Pressure (ml)
6 8 10 12 14 16 18 20 22 24 26
Stroke Volume (ml)
10
20
30
40
50
60
70
80
Healthy
CDM
PE
LV Volume (ml)
0 50 100 150 200 250 300
LV Pressure (mmHg)
0
20
40
60
80
100
120
140
Healthy
CDM
PE
ESPVR
ESPVR
ESPVR
EDPVR
EDPVR
EDPVR
RV Volume (ml)
0 20 40 60 80 100 120 140
RV Pressure (mmHg)
0
5
10
15
20
25
30
35
Healthy
CDM
PE
ESPVR
ESPVR
ESPVR
EDPVR
EDPVR
EDPVR
(A) (B)
(D)
(C)
ImpactofPericardialEffusiononCardiacMechanicsinPatientswithDilatedCardiomyopathy
643

K. D., Moulton, M. J., Ratcliffe, M. B., Pasque, M. K.,
2001. Mechanism underlying mechanical dysfunction
in the border zone of left ventricular aneurysm: a finite
element model study. Ann Thorac Surg 71:654-662.
Hayashida, W., Kumada, T., Nohara, R., Tanio, H.,
Kambayashi, M., Ishikawa, N., Nakamura, Y.,
Himura, Y., Kawai, C., 1990. Left ventricular regional
wall stress in dilated cardiomyopathy. Circulation
82:2075-2083.
Jhun, C. S., Wenk, J. F., Zhang, Z., Wall, S. T., Sun, K.,
Sabbah, H. N., Ratcliffe, M. B., Guccione, J. M.,
2010. Effect of adjustable passive constraint on the
failing left ventricle: a finite-element model study.
Ann Thorac Surg 89:132-137.
McCulloch, A., Waldman, L., Rogers, J., Guccione, J.,
1992. Large-scale finite element analysis of the
beating heart. Crit Rev Biomed Eng 20:427-449.
Mirhosseini, S. M., Fakhri, M., Mozaffary, A., Lotfaliany,
M., Behzadnia, N., Ansari Aval, Z., Ghiasi, S. M.,
Boloursaz, M. R., Masjedi, M. R., 2013. Risk factors
affecting the survival rate in patients with
symptomatic pericardial effusion undergoing surgical
intervention. Interact Cardiovasc Thorac Surg 16:495-
500.
Nakayama, Y., Shimizu, G., Hirota, Y., Saito, T., Kino,
M., Kitaura, Y., Kawamura, K., 1987. Functional and
histopathologic correlation in patients with dilated
cardiomyopathy: an integrated evaluation by
multivariate analysis. J Am Coll Cardiol 10:186-192.
Omens, J. H., MacKenna, D. A., McCulloch, A. D., 1993.
Measurement of strain and analysis of stress in resting
rat left ventricular myocardium. J Biomech 26:665-
676.
Quarterman, R. L., Moonly, S., Wallace, A. W., Guccione,
J., Ratcliffe, M. B., 2002. A finite element model of
left ventricular cellular transplantation in dilated
cardiomyopathy. ASAIO J 48:508-513.
Ratcliffe, M. B., Hong, J., Salahieh, A., Ruch, S., Wallace,
A. W., 1998. The effect of ventricular volume
reduction surgery in the dilated, poorly contractile left
ventricle: a simple finite element analysis. J Thorac
Cardiovasc Surg 116:566-577.
Swynghedauw, B., 1999. Molecular mechanisms of
myocardial remodeling. Physiol Rev 79:215-262.
Vetter, F. J., McCulloch, A. D., 2000. Three-dimensional
stress and strain in passive rabbit left ventricle: A
model study. Ann Biomed Eng 28:781-792.
Walker, J. C., Ratcliffe, M. B., Zhang, P., Wallace, A. W.,
Fata, B., Hsu, E. W., Saloner, D., Guccione, J. M.,
2005. MRI-based finite-element analysis of left
ventricular aneurysm. Am J Physiol-Heart C
289:H692-H700.
Wall, S. T., Walker, J. C., Healy, K. E., Ratcliffe, M. B.,
Guccione, J. M., 2006. Theoretical impact of the
injection of material into the myocardium - A finite
element model simulation. Circulation 114:2627-2635.
Wenk, J., Jhun, C.-S., Sun, K., Ratcliffe, M., Guccione, J.,
2010. Surgical Left Ventricular Remodeling
Procedures, in: Guccione, J. M., Kassab, G. S.,
Ratcliffe, M. B. (Eds.), Computational Cardiovascular
Mechanics. Springer US, pp. 197-210.
Zhong, L., Su, Y., Yeo, S. Y., Tan, R. S., Ghista, D. N.,
Kassab, G., 2009. Left ventricular regional wall
curvedness and wall stress in patients with ischemic
dilated cardiomyopathy. Am J Physiol Heart Circ
Physiol 296:H573-584.
SIMULTECH2013-3rdInternationalConferenceonSimulationandModelingMethodologies,Technologiesand
Applications
644
