
Achilles Tendinopathy is a Troublesome Sports-related Condition
Involving Blood Vessel Ingrowth into the Tendon Tissue
Studies on the Adjacent Plantaris Tendon and the Peritendinous Connective Tissue
Suggest that TNF-alpha can be Highly Involved in the Vascular and
Tissue Changes
C. Spang
1
, H. Alfredson
2
and S. Forsgren
1
1
Department of Integrative Medical Biology, Section for Anatomy, Umeå University, Umeå, Sweden
2
Department of Surgical and Perioperative Sciences, Sports Medicine, Umeå University, Umeå, Sweden
Keywords: Achilles Tendinopathy, Peritendinous Tissue, TNF-alpha, TNF Receptor, Blood Vessels, Plantaris Tendon.
Abstract: Achilles tendinopathy/tendinosis is a troublesome condition which is frequently occurring in response to
sports related activities. It can lead to an ending of the sport activity. There is evidence which shows that
ingrowth of blood vessels occurs from the peritendinous tissue. In well-established treatments the areas of
these vessels are targeted. In Achilles tendinosis there is frequently a coalescing of the plantaris tendon with
the Achilles tendon. TNF-alpha is known to be involved in blood vessel remodelling events and
angiogenesis. With these facts as background, the peritendinous connective tissue located inbetween the
plantaris and Achilles tendons and the plantaris tendon itself in cases with Achilles tendinosis were
evaluated concerning expression of TNF-alpha and TNF receptor II (TNFRII). It was found that there were
expressions of TNF-alpha in the numerous cells located in the peritendinous connective tissue and that the
very frequently occurring blood vessels located in this tissue as well as in the tendon tissue exhibited
marked TNFRII reactions. The tenocytes were shown to exhibit moderate TNF-alpha reactions and very
strong TNFRII reactions. The observations suggest that TNF-alpha is highly involved in the blood vessel
remodelling in tendinosis and that TNF-alpha also is involved in tenocyte function.
1 INTRODUCTION
Midportion Achilles tendinopathy, characterized by
chronic Achilles tendon pain, local swelling in the
midportion and loss of function (Khan et al., 1999),
is very frequent among sports athletes. It is assumed
that about 7-9% of professionals performing high
frequency of running and jumping suffer from this
condition (Cook et al., 2002); (Alfredson, 2003).
This makes up 6-18% of all injuries that happen in
running disciplines (Alfredson and Lorentzon,
2000); (Fahlström et al., 2002); (Schepsis et al.,
2002). It has also been shown that even moderate
activity in the form of badminton and track and field
activities can lead to the condition (Kvist, 1991);
(Fahlström et al., 2002). Repetitive strain is
considered to be the main risk factor (Kader et al.,
2002); (Paavola et al., 2002) but other aspects like
age, sex, training performance, muscle weakness and
lack of flexibility seem to be of importance as
background factors (Clement et al., 1984);
(Haglund-Akerlind and Eriksson, 1993); (Tuite et
al., 1997); (Hart et al., 1998); (Dudhia et al., 2007);
(Gaida et al., 2010). The precise underlying
mechanisms are still unclear.
Achilles tendinopathy is often called Achilles
tendinosis when besides pain, swelling and loss of
function, structural tissue changes can be observed
via ultrasound, MRI or histological evaluation (Khan
et al., 1999). A characteristic histological appearance
is the occurrence of an increased vascularization;
other changes are hypercellularity in early states,
decreasing cellularity in later states, cell rounding
and decreased matrix organization (Aström et al.,
1995); (Alfredson et al., 2003); (Riley, 2008). The
blood vessel ingrowth, which occurs from the
peritendinous connective tissue, is presumably of
great importance. It is thus to the regions with high
blood flow, as visualized via colour Doppler coupled
with ultrasonography, that treatments frequently are
45
Spang C., Alfredson H. and Forsgren S..
Achilles Tendinopathy is a Troublesome Sports-related Condition Involving Blood Vessel Ingrowth into the Tendon Tissue - Studies on the Adjacent
Plantaris Tendon and the Peritendinous Connective Tissue Suggest that TNF-alpha can be Highly Involved in the Vascular and Tissue Changes.
DOI: 10.5220/0004616500450050
In Proceedings of the International Congress on Sports Science Research and Technology Support (icSPORTS-2013), pages 45-50
ISBN: 978-989-8565-79-2
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

directed (Lind et al., 2006); (Alfredson, 2011a).
Currently used treatments for midportion
Achilles tendinopathy/tendinosis such as eccentric
training, injection treatments and traditional surgical
techniques have shown quite good clinical outcome.
However, there are still cases that have not been
found to be curable (Alfredson, 2011a).
Interestingly, it has been shown that 58 out of 73
(80%) Achilles tendinopathy tendons undergoing re-
operation with ultrasound+Doppler guided scraping
have an invaginated or “close by located” enlarged
plantaris tendon (Alfredson, 2011b). During Achilles
tendoscopy, it has also been noted that the plantaris
tendon can be seen to be affixed to the medial side
of the Achilles tendon in cases with tendinopathy
(Van Sterkenburg et al., 2011); (Van Sterkenburg
and Dijk, 2011).
The peritendinous tissue located outside the
Achilles tendon is likely to be of great importance in
the situations with tendinopathy and the curing of
this. It is thus known that this tissue represents a
dynamic and responsive region that markely adapts
to exercise (Kjaer, et al., 2000). It is e.g. shown that
there is an increase in bradykinin and adenosine
concentrations in the peritendinous tissue around the
Achilles tendon in response to exercise (Langberg et
al., 2002). In comparison, it has in recent studies
using a 14C bomb-pulse method been shown that the
tendon tissue itself has a poor regenerative capacity,
i.e. a lack of tissue renewal (Heinemeier et al.,
2013). An important part in the operation procedures
when the plantaris tendon is extirpated is a surgical
scraping procedure, the scraping being done for the
peritendinous connective tissue ventral to the
Achilles tendon (Alfredson, 2011c). The scraping is
guided by the evaluation of where the high blood
occurs, as visualized via ultrasound and laser
Doppler (Alfredson, 2011b).
There is a marked presence of peritendinous
connective tissue in the region between the plantaris
and Achilles tendons. As described above, the two
tendons can be very tightly connected via this tissue
in situations with Achilles tendinosis/tendinopathy.
Almost no attention has been paid to this tissue. The
information that exists says that that there is a
marked presence of blood vessels in the tissue, but
also frequent fibroblasts and to some extent
inflammatory cells as well (Spang et al., 2013).
As described above, the peritendinous connective
tissue outside tendinopathy tendons may be a very
important tissue. It is especially related to the basis
for the ingrowth of blood vessels that occurs from
this into the tendon tissue in tendinosis. Therefore,
this study was undertaken in studies when the
plantaris tendon is extirpated in the situation with
Achilles tendinosis. The signal substance system on
that was focused on was the TNF-alpha system. The
reason is that TNF-alpha is known to be involved in
blood vessel remodelling and angiogenesis (Baluk et
al., 2009); (Ligresti et al., 2011). We have also
previously observed that the tenocytes of the human
Achilles tendon show expression of TNF-alpha as
well as TNF receptors (Gaida et al., 2012).
Antibodies against TNF-alpha and TNF receptor
II (TNFRII) were applied. The hypothesis was that
the TNF-alpha system is involved in the processes in
tendinosis, including in the blood vessel
remodelling.
2 MATERIAL & METHODS
2.1 Individuals
Patients suffering from longterm pain (>3 months) in
the Achilles tendon midportion were included.
Examinations via ultrasound+Doppler showed
thickening, irregular tendon structure but also
hypoechoic regions and high blood flow localized
outside and inside the ventral midportion part
indicating Achilles tendinosis. The evaluated
material consisted of samples from 7 patients: 6 men
with a mean age of 40.2 years and 1 woman with an
age of 58. The samples conformed to specimens of
the plantaris tendon with attached peritendinous
connective tissue. For control purposes a specimen
from an individual without pain symptoms was
evaluated as well (female, 27 years).
2.2 Sampling
During the surgery, the patients were kept under
local anaesthesia (Pilokainhydrochloride 4-5 ml, 10
mg/ml, Astra Zeneca, Södertälje, Sweden). The
procedure was as follows: Via a short longitudinal
skin incision on the medial side the Achilles tendon
was visualized. The plantaris tendon was in these
cases discovered to lie very close to the Achilles
tendon’s medial and ventral part. In some cases it
was even seen to be invaginated. Then the plantaris
tendon was carefully freed distally and proximally
and finally cut at both ends. The tendon tissue was
accompanied by closely attached peritendinous
tissue. The Achilles tendon was thereafter “scraped”
in the regions with high blood flow on the ventral
side according to currently outlined procedures
(Alfredson, 2011c). For control purposes a plantaris
tendon from healthy individual (female, 27 years)
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
46

was taken as well (c.f. above). Ultrasound+Doppler
showed no pathological features in this case. Due to
ethical reasons the obtaining of control tissue was
restricted in this case.
The study protocol was approved by the
Regional Ethical Board in Umeå (dnr 04-157M;
2011-83-32M). The experiments were conducted
according to the principles expressed in the
Declaration of Helsinki.
2.3 Fixation, Sectioning and Staining
for Morphology
The procedures for fixation and sectioning are in
accordance with previously described procedures for
tendon specimens (Spang et al., 2013); (Gaida et al.,
2012). For demonstration of morphology, sections
were stained with haematoxylin and eosin (H&E).
2.4 Immunofluorescence Processing
Immunostainings for detecting immunoreactions for
TNF-alpha and TNFRII were performed. The
procedures conform to those previously used in our
laboratory for the demonstration of these factors
(Gaida et al., 2012). As secondary antiserum,
fluorescein isothiocyanate (FITC)-conjugated
AffiniPure donkey antigoat IgG (1:100) (code no:
705-095-003, Jackson Immune Research, West
Grove, Pa., USA) was used. Examination was
carried out in a Zeiss Axioscope 2 plus microscope
equipped with epifluorescent technique and an
Olympus DP70 digital camera. The antibodies used
are goat polyclonal antibodies from Santa Cruz
Technology (Santa Cruz, CA, USA). The antibody
for detecting TNF-alpha (L-19, code no.: SC-1350)
was used at a dilution of 1:100 in PBS. The antibody
against TNFRII (C-20, code no.: SC-1074) is
primarily targeted to the C-terminus of human
TNFRII. It was diluted 1:50 in PBS. For further
information on the antibodies, staining procedures
and control stainings, see Gaida et al., (2012).
3 RESULTS
3.1 Morphology
Microscopical analysis of the Htx-Eosin treated
sections showed that the samples contained tendon
tissue and also parts of the peritendinous connective
tissue. There was a large number of fine and large
blood vessels in the peritendinous connective tissue
(Figure 1). There were also very frequent cells in the
tissue. The cells have in a previous study been found
to be stained with antibodies against fibroblast
marker or macrophage marker (Spang et al., 2013).
There was also a large number of blood vessels
within the tendon tissue Compared to the control
specimen the loose connective tissue from tendinosis
patients contained a much higher number of blood
vessels and more cells.
Figure 1: Peritendinous connective tissue between the
plantaris and the Achilles tendon stained for haematoxylin
and eosin. There is a marked presence of blood vessels.
3.2 Immunohistochemistry
3.2.1 TNF-alpha
Immunoreactions for TNF-alpha could be observed
in cells in the peritendinous connective tissue
(Figure 2a) and in tenocytes in the tendon proper
(Figure 2b) in the tendinosis specimens. Weak
reactions could be detected in the walls of blood
vessels located in the tendon proper (Figure 2c) and
in the peritendinous connective tissue (Figure 2d) in
theses specimens. The immunoreactions in the cells
in the peritendinous connective tissue were very
finely point-like. Immunoreactions could also be
observed in the occasionally seen cells in the
peritendinous tissue, to some extent in blood vessel
walls and in tenocytes in the control specimen (not
illustrated).
3.2.3 TNFRII
TNFRII immunoreactions could be detected in the
same type of structures as referred to above in the
tendinosis specimens, namely the cells in the
peritendinous connective tissue, the blood vessel
walls and in tenocytes (Figure 3a-d). The reactions
were strong and seen as small bright dots.
Particularly, the blood vessel reactions were very
marked.
AchillesTendinopathyisaTroublesomeSports-relatedConditionInvolvingBloodVesselIngrowthintotheTendonTissue
-StudiesontheAdjacentPlantarisTendonandthePeritendinousConnectiveTissueSuggestthatTNF-alphacanbeHighly
InvolvedintheVascularandTissueChanges
47
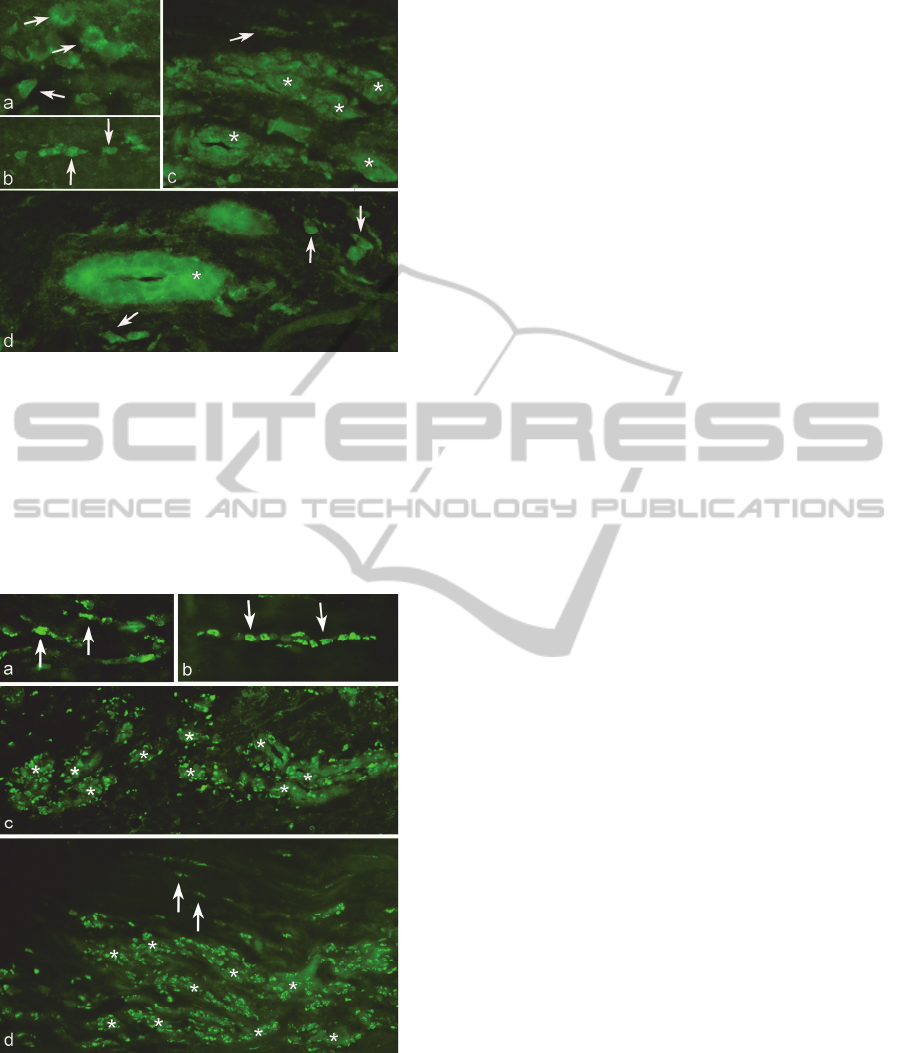
Figure 2: Immunolabelling for TNF-alpha. Plantaris
tendon with attached peritendinous connective tissue is
shown. Immunoreactions are observed in cells in the
peritendinous connective tissue (a, arrows). Tenocytes in
the tendon proper do also show specific reactions (b,
arrows). Blood vessels in the tendon proper (c) and in the
peritendinous connective tissue (d) are weakly positive for
TNF-alpha (asterisks). Arrows at immunoreactive tenocyte
in (c) and at immunoreactive cells in the peritendinous
connective tissue in (d).
Figure 3: Immunolabelling for TNFRII. Plantaris tendon
with attached peritendinous connective tissue is shown.
Immunoreactions are seen in the cells in the peritendinous
connective tissue (a, arrows) and in tenocytes in the
tendon proper (b,d arrows). Furthermore very marked
immunoreactions are seen in the walls of blood vessels in
both the peritendinous connective tissue (c, asterisks) and
the tendon proper (d, asterisks).
There were thus areas in the peritendinous
connective tissue and in tendon tissue proper that
exhibited widespread TNFRII reactions (Fig.3c,d).
Immunoreactions were also noted for the blood
vessel walls and to some extent for the tenocytes and
the cells in the peritendinous connective tissue in the
control specimen.
4 DISCUSSION
These evaluations show for the first time the
occurence of TNF-alpha and TNFRII immuno-
reactions in the plantaris tendon and in the
peritendinous connective tissue between the
plantaris tendon and the Achilles tendon in cases
with Achilles tendinosis. It is evident that there is a
local TNF-alpha production in cells in this tissue and
that TNF-alpha is involved in the blood flow
regulation of the tissue. There were thus very
marked TNFRII reactions in the vessel walls. To
some extent there were similar reaction patterns in
the control specimen. However, it should be
underscored that the vessels and the cells in the
peritendinous connective tissue were much fewer
than what was case for the tendinosis specimens.
Due to ethical reasons, the obtaining of control
samples was restricted to one individual.
Concerning the marked TNFRII immuno-
reactions seen for blood vessel walls it is noteworthy
that this TNF receptor is shown to enhance
angiogenesis under low oxygen conditions (Luo et
al., 2006). TNF-alpha is on the whole known to be
involved in blood vessel regulation. It is e.g. shown
that TNF-alpha is involved as an early component in
the cascade leading to angiogenesis in response to
aortic injury (Ligresi et al., 2011). The origin of the
TNF-alpha in this case was macrophages (Ligresi et
al., 2011). Renal ischemia is shown to be
accompanied by increased expressions of TNF-alpha
and TNF receptors, an increased expression of
TNFRII being observable for the renal arteries and
the neuroretina (Gesslein et al., 2010).
TNFRII, as well as TNF-alpha, immuno-
reactions were also observed for tenocytes of the
plantaris tendons. The situation is thus the same as
was previously observed for the human Achilles
tendon (Gaida et al., 2012). This can imply that
autocrine/paracrine TNF-alpha effects occur for the
tenocytes, effects which can be related to trophic
functions. In accordance with this suggestion, it is
known that binding at TNFRII is related to tissue
repair, growth-modulating effects and differentiation
(Ihnatko and Kubes, 2007). Due to the “compression
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
48

theory” the compressive forces on the peritendinous
tissue can be very strong in Achilles tendinosis
(Cook and Purdam, 2009). Therefore, tissue repair,
growth-modulating influences and differentiation
might play key roles during the tendinosis condition.
TNF-alpha blockers have been tested for patients
suffering from chronic Achilles tendinopathy
(Fredberg and Ostgaard, 2009). The clinical
implication however is still unclear. The results of
the present study indicate that further studies should
be undertaken concerning the use of anti-TNF
treatments.
In conclusion it is here shown that there is a
marked presence of the TNF-alpha system in the
situation with plantaris tendon involvement in
Achilles tendinosis. TNF-alpha is produced in the
peritendinous connective tissue and may be highly
involved in the blood vessel remodelling as well as
for tenocyte function. These findings stress that the
TNF-alpha system might be an important system in
a condition that is frequently involved for sports
active persons, namely Achilles tendinopathy/
tendinosis.
ACKNOWLEDGEMENTS
The authors acknowledge Ms Ulla Hedlund for
excellent technical services. Financial support was
obtained from the Faculty of Medicine at Umeå
University and the Swedish National Centre for
Research in Sports.
REFERENCES
Alfredson, H. & Lorentzon, R., 2000. Chronic Achilles
tendinosis: recommendations for treatment and
prevention. Sports Medicine, 29(2), 135-146.
Alfredson, H., 2003. Chronic midportion Achilles
tendinopathy: an update on research and treatment.
Clinical Sports Medicine, 22(4), 727-741.
Alfredson, H., Ohberg, L. & Forsgren, S., 2003. Is
vasculo-neural ingrowth the cause of pain in chronic
Achilles tendinopathy? An investigation using
ultrasonography and color Doppler,
immunehistochemistry, and diagnostic injections.
Knee Surgery, Sports, Traumatology, Arthroscopy,
11(5), 334-338.
Alfredson, H., 2011a. Where to now with Achilles tendon
treatment? British Journal of Sports Medicine, 45,
407-410.
Alfredson, H., 2011b. Midportion Achilles tendinosis and
the plantaris tendon. British Journal of Sports
Medicine, 45(13), 1023-1025.
Alfredson, H., 2011c. Ultrasound and Doppler-guided
mini-surgery to treat midportion Achilles tendinosis:
results of a large material and a randomized study
comparing two scraping techniques. British Journal
of Sports Medicine, 45, 407-410.
Alfredson, H., Spang, C. & Forsgren, S., 2013. Unilateral
surgical treatment for patients with midportionAchilles
tendinopathy may result in bilateral recovery, British
Journal of Sports Medicine, [Epub ahead of print].
Andersson, G. et al., 2007. Nerve-related characteristics of
ventral paratendinous tissue in chronic Achilles
tendinosis. Knee Surgery, Sports, Traumatology and
Arthroscopy, 15, 1272-1279.
Andersson, G. et al., 2008. Presence of substance P and
the neurokinin-1 receptor in tenocytes of the human
Achilles tendon. Regulatory Peptides, 150(1-3), 81-87.
Aström, M. & Rausing, A., 1995. Chronic Achilles
tendinopathy. A surgery of surgical and
histopathological findings. Clinical Orthopaedics and
Related Research, 316, 246-252.
Baluk, P. et al., 2009, TNF-alpha drives remodeling of
blood vessels and lymphatics in sustained airway
inflammation in mice. Journal of Clinical
Investigations, 119, 2954-2964.
Bjur, D. et al., 2008a. Presence of a non-neuronal
cholinergic system and occurrence of up- and down-
regulation in expression of M2 muscarinic
acetylcholine receptors: new aspects of im-portance
regarding Achilles tendon tendinosis (tendinopathy).
Cell and Tissue Research, 331(1), 385-400.
Bjur, D. et al., 2008b. Immunohistochemical and in situ
hybridization observations favour a local
catecholamine production in the human Achilles
tendon. Histology and Histopathology, 23(2), 197-208.
Clement, D. B., Taunton, J. E. & Smart, G. W., 1984.
Achilles tendinitis and peritendinitis: etiology and
treatment. American Journal of Sports Medicine,
12(3), 179-184.
Cook, J. L., Khan, K. H. & Purdam, C., 2002. Achilles
tendinopathy.
Manual Therapy, 7(3), 121-130.
Cook, J. & Purdam, C., 2009. Is tendon pathology a
continuum? A pathology model to explain the
clinical presentation of load-induced tendinopathy.
British Journal of Sports Medicine, 43(6), 409-16.
Danielson, P., 2009. Reviving the "biochemical"
hypothesis for tendinopathy: new findings suggest the
involvement of locally produced signal substances.
British Journal of Sports Medicine, 43(4), 265-268.
Dudhia, J. et al., 2007. Aging enhances a mechanically-
induced reduction in tendon strength by an active
process involving matrix metalloproteinase activity.
Aging Cell, 6(4), 547-556.
Fahlström, M., Lorentzon, R. & Alfredson, H., 2002.
Painful conditions in the Achilles tendon region in
elite badminton players. American Journal of Sports
Medicine, 30(1), 51-54.
Fredberg, U & Ostgaard, R., 2009. Effect of ultrasound-
guided, peritendinous injections of adalimumab and
anakinra in chronic Achilles tendinopathy: a pilot
study. Scandinavian Journal of Medicine and Science
in Sports, 19(3), 338-344.
AchillesTendinopathyisaTroublesomeSports-relatedConditionInvolvingBloodVesselIngrowthintotheTendonTissue
-StudiesontheAdjacentPlantarisTendonandthePeritendinousConnectiveTissueSuggestthatTNF-alphacanbeHighly
InvolvedintheVascularandTissueChanges
49

Gaida, J. E. et al., 2010. Asymptomatic Achilles tendon
pathology is associated with a central fat distribution
in men and a peripheral fat distribution in women: a
cross sectional study of 298 individuals. BMC
Musculoskeletal Disorders, 11, 41.
Gaida, J. et al., 2012. Evidence of the TNF-α system in the
human Achilles tendon: expression of TNF-α and TNF
receptor at both protein and mRNA levels in the
tenocytes. Cells Tissues Organs, 196(4), 339-352.
Gesslein, B. et al., 2010. Tumor necrosis factor and its
receptors in the neuroretina and retinal vasculature
after ischemia-reperfusion injury in the pig retina.
Molecular Vision, 16, 2317-2327.
Haglund-Akerlind, Y. & Eriksson, E., 1993. Range of
motion, muscle torque and training habits in runners
with and without Achilles tendon problems. Knee
Surgery, Sports, Traumatology, Arthroscopy, 1(3-4),
195-199.
Hansson, M. & Forsgren, S., 1995. Immunoreactive atrial
and brain natriuretic peptides are co-localized in
Purkinje fibres but not in the innervation of the bovine
heart conduction system. Histochemical Journal,
27(3), 222-230.
Hart, D. A. et al., 1998. Gender and neurogenic variables
in tendon biology and repetitive motion disorders.
Clinical Orthopaedics and Related Research, (351),
44-56.
Heinemeier, K. M. et al., 2013. Lack of tissue renewal in
human adult Achilles tendon is revealed by nuclear
bomb 14C. FASEB Journal, Feb 19. Epub ahead of
print.
Hershey, A. D. & Krause, J. E., 1990. Molecular
characterization of a functional cDNA encoding the rat
substance P receptor. Science, 247, 4945, 958–962.
Ihnatko, R. and Kubes, M., 2007. TNF signaling: early
events and phosphorylation. General Physiology and
Biophysics, 26(3), 159-67.
Kader, D. et. al, 2002. Achilles tendinopathy: some
aspects of basic science and clinical management.
British Journal of Sports Medicine, 36(4), 239-249.
Khan, K. M. et al., 1999. Histopathology of common
tendinopathies. Update and implications for clinical
management. Sports Medicine, 27(2), 393-408.
Kjaer, M. et al., 2000. In vivo studies of peritendinous
tissue in exercise. Scandinavian Journal of Medicine
and Science in Sports, 10, 326-331. &
Langberg, H. et al., 2002. Exercise-induced increase in
interstitial bradykinin and adenosine concentrations in
skeletal muscle and peritendinous tissue in humans.
Journal of Physiology, 542, 977-983.
Ligresi, G. et al., 2011. Macrophage-derived tumor
necrosis factor-alpha is an early component of the
molecular cascade leading to angiogenesis in response
to aortic injury. Arteriosclerosis, Thrombosis, and
Vascular Biology, 31, 1151-1159.
Lind, B., Ohberg, L. & Alfredson, H., 2006. Sclerosing
polidocanol injections in mid-portion Achilles
tendinosis: remaining good clinical results and
decreased tendon thickness at 2-year follow-up. Knee
Surgery, Sports, Traumatology and Arthroscopy,
14(12), 1327-1332.
Luo, D. et al., 2006. Differential functions of tumor
necrosis factor receptor 1 and 2 signaling in ischemia-
mediated arteriogenesis and angiogenesis. American
Journal of Pathology, 169(5), 1886-1898.
Miller, N. L. et al., 2010. Inflammation is present in early
human tendinopathy. American Journal of Sports
Medicine, 38(10), 2085-2091.
Nishida, T., 2005. Neurotrophic mediators and corneal
wound healing. The Ocular Surface, 3(4), 194-202
Paavola et al., 2002. Current concepts review Achilles
tendinopathy. Journal of Bone and Joint Surgery
(American Volume), 84A(11), 2062-2076.
Patel, T. et al., 2003. Substance P induces interleukin-8
secretion from human dental pulp cells. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod, 96(4), 478-
485
Riley, G., 2008. Tendinopathy – from basic science to
treatment. Nature Clinical Practice Rheumatology,
4(2), 82-89
Rolf, C. & Movin, T., 1997. Etiology, histopathology, and
outcome of surgery in achillodynia. Foot & Ankle
International, 18(9), 565-569.
Schepsis, A. A., Jones, H. & Haas, A. L., 2002. Achilles
tendon disorders in athletes. American Journal of
Sports Medicine, 30(2), 287-305.
Scott, A., Alfredson, H. & Forsgren, S., 2008. VGluT2
expression in painful Achilles and patellar tendinosis:
evidence of local glutamate release by tenocytes.
Journal of Orthopaedic Research, 26(5), 685-692.
Spang, C. et al., 2013. The plantaris tendon in association
with mid-portion Achilles tendinosis - Tendinosis-like
morphological features and presence of a non-neuronal
cholinergic system. Histology & Histopathology,
28(5), 623-32.
Tuite, D. J., Renstrom, P. A. & O’Brien, M., 1997. The
aging tendon. Scandinavian Journal of Medicine &
Science in Sports, 7(2), 72-77.
Van Sterkenburg, M.N., 2011. The plantaris tendon and a
potential role in mid-portion Achilles tendinopathy: an
observational anatomical study. Journal of Anatomy,
218, 336-341.
Van Sterkenburg, M. N. & Van Dijk, C. N., 2011. Mid-
portion Achilles tendinopathy: why painful? An
evidence-based philosophy. Knee Surgery, Sports,
Traumatology and arthrosopy, 20(8), 1653-1654.
Van Sterkenburg, M. N., Kerkhoffs, G. & Van Dijk, N.,
2011. Good outcome after stripping the plantaris
tendon in patients with chronic mid-portion Achilles
tendinopathy. Knee Surgery, Sports, Traumatology
and Arthroscopy, 19(8), 1362-1366.
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
50
