
A Viscoelastic Model for Glioma Growth
J. R. Branco
1
, J. A. Ferreira
2
and P. de Oliveira
2
1
CMUC, Department of Physics and Mathematics, Coimbra Institute of Engineering, Coimbra, Portugal
2
CMUC, Department of Mathematics, University of Coimbra, Coimbra, Portugal
Keywords:
Glioma, Viscoelastic Behaviour, Mathematical Model, Numerical Simulation.
Abstract:
In this paper we propose a mathematical model to describe the evolution of glioma cells in the brain taking
into account the viscoelastic properties of brain tissue. The mathematical model is established considering
that the glioma cells are of two phenotypes: migratory and proliferative. The evolution of the migratory cells
is described by a diffusion-reaction equation of non Fickian type deduced considering a mass conservation
law with a non Fickian migratory mass flux. The evolution of the proliferation cells is described by a reaction
equation. Numerical simulations that illustrate the behaviour of the mathematical model are included.
1 INTRODUCTION
Cancer is a complex disease which leads to the un-
controlled growth of abnormal cells, destruction of
normal tissues and invasion of vital organs. There
are different stages at tumor development of vary-
ing duration, starting from genetic changes at the cell
level and finishing with detachment of metastases and
invasion. Tumor cell transport and proliferation are
the main contributors to the malignant dissemination
((Swanson et al., 2003)).
Extensive research has been done to model can-
cerous growth, specially on solid tumors, in which
growth primarily comes from cellular proliferation.
It is far beyond the aim of the present paper to list
exhaustively the many significant contribution in the
topic. References (Fedotov and Iomin, 2007), (Giese
et al., 1996), (Habib et al., 2003), (Harpold et al.,
2007), (Mur, 2002), (Swanson et al., 2000), (Swan-
son et al., 2003) and the references therein represent
some of these contributions.
However the understanding of malignant gliomas
is much less complete, mostly because gliomas prolif-
erate as solid tumors and invade the surrounding brain
parenchyma actively. Proliferation and specially mi-
gration of gliomas represent a very challenging prob-
lem from mathematical viewpoint.
Gliomas are diffusive and highly invasivebrain tu-
mors accounting for about 50% of all primary brain
tumors and, unfortunately, the prognosis for patients
with gliomas is very poor. Median untreated survival
time for high grade gliomas ranges from 6 months to
1 year and even lower grade gliomas can rarely be
cured. Theorists and experimentalists believe that in-
efficiency of treatments results from the high mobility
of glioma cells. Additionally gliomas can exhibit very
high proliferation rates.
Cancer research has been a fertile ground for
mathematical modeling, beginning with the early con-
cept of simple exponential growth of solid tumors
doubling at a constant rate. The introduction of lo-
gistic or gompertzian growth (there is increased dou-
bling time and decreased growth fraction as a func-
tion of time) allowed to slow the growth in the later
stages. With the recognition that tumor cells might
spread outside the grossly visible mass, invading lo-
cally and metastasizing distantly, and that some cells
die during the development process, the mathematical
concepts necessarily became more complicated than
those used in the original simple models for solid tu-
mors.
The initial answer to the question of how to mea-
sure the growth of an infiltrating glioma was provided
by Murray in the early 90s ((Mur, 2002)). He formu-
lated the problem as a conservation law where the rate
of change of tumor cell population results from mo-
bility and net proliferation of cells. An equation of
type
∂c
∂t
+ ∇.J
F
= f(c)in Ω ×(0, ∞) (1)
was used, where Ω ⊂R
n
,n = 1,2,3, is the glioma do-
main, c(x,t) denotes the tumor cell density at location
x and time t, f(c) denotes net proliferation of tumor
cells, and ∇ defines the spatial gradient operator. Un-
689
R. Branco J., Ferreira J. and de Oliveira P..
A Viscoelastic Model for Glioma Growth.
DOI: 10.5220/0004632406890695
In Proceedings of the 3rd International Conference on Simulation and Modeling Methodologies, Technologies and Applications (BIOMED-2013), pages
689-695
ISBN: 978-989-8565-69-3
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

der the assumption of the classical Fick’s law for the
mass flux J
F
J
F
= −D∇c, (2)
where D is the diffusion tensor, the model can be writ-
ten as
∂c
∂t
= ∇.(D∇c) + f(c) in Ω ×(0, ∞). (3)
The mathematical model is complemented by bound-
ary conditions which impose no migration of cells be-
yond the brain boundary, that is,
J
F
.η = 0,
on the boundary, where η denotes the exterior unit
normal to the brain region, and by initial conditions
c(x,0) = c
0
(x),x ∈ Ω, where c
0
defines the initial
spatial distribution of malignant cells.
Tumor growth is generally assumed to be ex-
ponential, so that the cell growth term is given by
f(c) = ρc, where the net proliferation rate ρ is con-
stant. However, logistic and gompertzian growths
have been considered but found to be unnecessary
in the time frames considered for gliomas ((Harpold
et al., 2007)). To apply the modeling approach to spe-
cific patients, a more realistic look at the brain geome-
try and structure was necessary. Swanson et al. intro-
duced in (Swanson et al., 2000) the complex geome-
try of the brain and allowed diffusion to be a function
of the spatial variable to reflect the observation that
glioma cells exhibit higher motility in the white mat-
ter than in grey matter.
Finally we observe that the most popular treat-
ments used to combat gliomas are chemotherapy and
radiation. Mathematical models to describe the effect
of the previous treatments were proposed in the liter-
ature. Without being exhaustive we mention (Rockne
et al., 2009) and (Tracqui et al., 1995).
The partial differential equation (3), of parabolic
type,was established combing the mass conservation
law (1) with Fick’s law (2) for mass flux. It is
well known that, in this case, if a sudden change
on the cell concentration takes place somewhere in
the space, it will be felt instantaneously everywhere
this means that Fickian approach gives rise to infi-
nite speed of propagation which is not physically ob-
servable. To avoid the limitation of Fickian mod-
els an hyperbolic correction has been proposed in
different contexts (see (Edwards and Cohen, 1995),
(Joseph and Preziosi, 1989), (Fedotov, 1998), (Fedo-
tov, 1999), (Hassanizadeh, 1996), (Neuman and Tar-
takovsky, 2009) and the references cited in those pa-
pers).
The aim of the present paper is the establishment
of a class of non Fickian models that take into ac-
count the viscoelastic behavior of the brain tissue.
The paper is organized as follows. Since the brain tis-
sue presents a viscoelastic behaviour that can be de-
scribed by the Voigt-Kelvin model (see for instance
(G.Franceschini, 2006), (Humphrey, 2003), (Mehra-
bian and Abousleiman, 2011)), we present in Sec-
tion 2 a class of non Fickian models to describe the
space and time evolution of glioma cancer cells con-
structed by combining the diffusion process with the
viscoelastic properties of the brain tissue. In Section
3 we study the behaviour of the glioma mass. In Sec-
tion 4 we introduce the numerical method that will be
used to obtain numerical approximations for the den-
sity of proliferation and migratory glioma cells. Plots
illustrating the evolution of gliomas are included in
Section 5. Finally, in Section 6 we present some con-
clusions.
2 A VISCOELASTIC MODEL
The class of non Fickian models that we present in
what follows is established taking into account the
viscoelastic nature of the brain tissue. Following (Ed-
wards and Cohen, 1995), (Edward and Cohen, 1995),
(Edwards, 1996), (Edwards, 2001) and (Shaw and
Whiteman, 1998), if a diffusion process occurs in a
medium that has a viscoelastic behaviour, then this
behaviour should be included in the diffusion equa-
tion which leads to a modified diffusion equation
∂c
∂t
= ∇.(D∇c) + ∇.(D
v
∇σ) + f(c) in Ω×(0,∞),
(4)
where σ represents the stress exerted by the brain tis-
sue on the tumor cells.
We assume that the viscoelastic behaviour of the
brain tissue is described by
∂σ
∂t
+ βσ = α
1
ε+ α
2
∂ε
∂t
, (5)
where ε stands for the strain. Equation (5) is based on
a mechanistic model which is represented by a spring
(restorativeforce component) and a dashpot (damping
component) in parallel connected with a free spring.
In (5) the viscoelastic characteristic time β is given
by β =
E
0
+E
1
µ
1
, and α
1
=
E
0
E
1
µ
1
, α
2
= E
0
where E
1
is
the Young modulus of the spring element, µ
1
repre-
sents the viscosity and E
0
stands for the Young mod-
ulus of the free spring (see (G.Franceschini, 2006),
(Humphrey, 2003), (Mehrabian and Abousleiman,
2011)).
Equation (5) leads to the following expression for
σ
σ(t) =
Z
t
0
e
−β(t−s)
α
1
ε(s) + α
2
∂ε
∂t
(s)
ds+ e
−βt
σ(0).
(6)
SIMULTECH2013-3rdInternationalConferenceonSimulationandModelingMethodologies,Technologiesand
Applications
690

If we assume that the strain ε satisfies ε = λc
where λ is a positive constant(see (Edwards and Co-
hen, 1995), (Edward and Cohen, 1995), (Edwards,
1996) and (Edwards, 2001)) we obtain from (4) and
(6) an integro-differential equation of type
∂c
∂t
= ∇.(D∇c) +
Z
t
0
k
er
(t −s)∇.(D
v
∇c(s))ds
+ f(c) in Ω×(0,∞),
(7)
where k
er
(s) = e
−βs
.
To establish a mathematical model to describe the
space-time evolution of the gliomas some medical
information is needed. According to (Fedotov and
Iomin, 2007) and (Fedotov and Iomin, 2008) the fol-
lowing assumptions are considered in our model:
• the glioma cells are of two phenotypes - prolifer-
ation (state 1) and migratory (state 2);
• in state 1 (migratory phenotype) the cells ran-
domly move but there is no cell fission;
• in state 2 (proliferation phenotype) the cancer
cells do not migrate and only proliferation takes
place with rate ρ;
• a cell of type 1 remains in state 1 during a time
period and then switches to a cell of type 2;
• β
1
is the switching rate from state 1 to 2;
• a cell of type 2 remains in state 2 during a time
period and then switches to a cell of type 1;
• β
2
is the switching rate from state 2 to 1.
Let u(x,t) and v(x,t) represent the density of mi-
gratory and proliferation cells at x and t, respectively.
The dynamics of glioma cells is then described by
∂u
∂t
= ∇.(D∇u) +
Z
t
0
k
er
(t −s)∇.(D
v
∇u(s))ds
−β
1
u+ β
2
v,
∂v
∂t
= ρv+ β
1
u−β
2
v,
in Ω×(0,T],
(8)
where D and D
v
denote square matrices of order n and
β
1
is the switching rate from migratory phenotype to
proliferation phenotype and β
2
is the switching rate
from proliferation phenotype to migratory phenotype.
The set of equations (8) is complemented with initial
conditions
u(0) = u
0
,v(0) = v
0
in Ω,
and boundary conditions
J.η = 0 on ∂Ω, (9)
where ∂Ω denotes the boundary of Ω, η represents
the exterior unit normal and the non Fickian flux J is
given by
J(t) = −D∇u(t) −
Z
t
0
e
−β(t−s)
D
v
∇u(s)ds.
Condition (9) means that the glioma is located inside
of the brain and the cancer cells do not cross the pia
mater.
We observe that the first equation of (8) can be de-
duced considering the mass conservation law (1) and
a modified Fick’s law for the mass flux. In fact, if we
assume that the mass flux J has two contributions, a
Fickian and a non Fickian, that is
J = J
F
+ J
NF
,
where J
NF
is given by
J
NF
(t) = −
Z
t
0
e
−β(t−s)
D
v
∇u(s)ds, (10)
we obtain (8) from (1). We note that (10) satisfies the
following IVP
∂J
NF
∂t
+ βJ
NF
= −D
v
∇uin Ω×(0,+∞),
J
NF
(0) = 0in Ω,
(11)
where the first equation of (11) is a first order approx-
imation of the equality
J
NF
(x,t +
1
β
) = −
1
β
D
v
∇u(x,t). (12)
This equation establishes that non Fickian mass flux
at time t +
1
β
, where
1
β
is the relaxation time, is re-
lated with the gradient of the concentration u at a pre-
vious time. This observation means that system (8)
incorporates a certain memory effect induced by the
behaviour of migratory cells.
3 QUALITATIVE BEHAVIOUR
In what follows we assume that D = [d
ij
] and D
v
=
[d
v,i j
] are diagonal matrices with diagonal entries d
i
and d
v,i
such that
0 < d
i
,d
v,i
in
Ω,i = 1,. . .,n. (13)
Let M (t) be the mass of glioma cells in Ω,
M
1
(t) =
Z
Ω
(u(t) + v(t))dx.
AViscoelasticModelforGliomaGrowth
691

We study in what follows the behaviour of M
1
(t).
We start by remarking that
M
′
1
(t) =
Z
Ω
∂u
∂t
(t) +
∂v
∂t
(t)
dx. (14)
As u and v are defined by the system of equations (8),
from (14) we obtain
M
′
1
(t) =
Z
Ω
(−∇.J(t) + ρv(t))dx,
that leads to
M
′
1
(t) = −
Z
∂Ω
J(t).ηds+ ρ
Z
Ω
v(t)dx. (15)
From (9) we conclude that
M
′
1
(t) = ρ
Z
Ω
v(t)dx,
which means that the instantaneous time variation of
the cancer mass depends only on the mass of the pro-
liferation cells and on the proliferation rate ρ. Assum-
ing the positivity of u, we finally obtain the upper
bound
M
1
(t) ≤ e
ρt
M
1
(0). (16)
To avoid the positivity assumption on u we estab-
lish in what follows an upper bound for
M
2
(t) = ku(t)k
2
+ kv(t)k
2
,
where k.k denotes the usual L
2
norm and which is
induced by the usual L
2
inner product (.,.).
We have
1
2
M
′
2
(t) = (
∂u
∂t
(t),u(t)) + (
∂v
∂t
(t),v(t))
As (8) holds we obtain
1
2
M
′
2
(t) =
Z
∂Ω
−J(t).ηu(s)ds−k
√
D∇u(t)k
2
−((
Z
t
0
k
er
(t −s)D
v
∇u(s)ds,∇u(t)))
−β
1
ku(t)k
2
+ (−β
2
+ ρ)kv(t)k
2
+(β
1
+ β
2
)(u(t),v(t)),
(17)
where the inner product in L
2
(Ω) ×L
2
(Ω) is denoted
by ((.,.)) and represents k.k the induced norm.
Considering the boundary condition (9), the Cauchy-
Schwarz inequality and the following equality
d
dt
k
Z
t
0
k
er
(t −s)
p
D
v
∇u(s)dsk
2
= 2((
Z
t
0
k
er
(t −s)D
v
∇u(s)ds,∇u(t)))
−2βk
Z
t
0
k
er
(t −s)
p
D
v
∇u(s)dsk
2
,
we deduce from (17) that
E
′
(t) ≤ max{β
2
−β
1
,β
1
−β
2
+ 2ρ,−2β}E(t),t > 0,
(18)
where
E(t) = M
2
(t) + k
Z
t
0
k
er
(t −s)
p
D
v
∇u(s)dsk
2
.
Inequality (18) leads to
M
2
(t) ≤ e
max{β
2
−β
1
,β
1
−β
2
+2ρ,−2β}t
M
2
(0). (19)
The upper bound for the glioma mass defined by in-
equality (19) depends on the parameters of the model:
the switching rate β
1
from migratory state to prolifer-
ation state; the switching rate β
2
from proliferation
state to migratory state, the proliferation rate ρ of the
cells of type 2 and the viscoelastic characteristic time
β. If β
2
= β
1
then the upper bound is e
2ρt
M
2
(0) which
is analogous to the one obtained for M
1
(t) with arbi-
trary β
1
,β
2
. Moreover, if 0 < β
2
−β
1
< ρ, then the
upper bound is e
(2ρ−(β
2
−β
1
))t
M
2
(0) being the ampli-
fication factor e
(2ρ−(β
2
−β
1
))t
greater than e
ρt
obtained
for M
1
(t). However as expected, under these assump-
tions, we can not select parameter β
2
,β
1
,ρ such that
the increasing of migratory cells is bounded.
We remark that inequality (19) allow us to con-
clude the stability of the proposed mathematical
model with respect to perturbations of the initial con-
ditions.
4 NUMERICAL METHOD
We assume in what follows that n = 2, Ω is the square
[0,L] ×[0, L] and H = (h, k) with h > 0,k > 0. In
Ω
we introduce the spatial grid
Ω
H
= {(x
1,i
,x
2, j
),i = 0, . .. ,N
h
, j = 0,. . ., N
k
}
where x
1,i
= x
1,i−1
+ h, i = 1,...,N
h
, x
0
= 0, x
1,N
h
=
L, and x
2, j
= x
2, j−1
+ k, j = 1, .. ., N
k
, x
2,0
=
0, x
2,N
k
= L. By ∂Ω
H
we represent the set of boundary
points. We introduce the following auxiliary points
x
1,−1
= x
1,0
−h, x
1,N
h
+1
= x
1,N
h
+ h,
and
x
2,−1
= x
2,0
−k, x
2,N
k
+1
= x
2,N
k
+ k.
By ∂Ω
′
H
we denote the following set of auxiliary
points
∂Ω
′
H
= {(x
1,−1
,x
2, j
),(x
1,N
h
+1
,x
2, j
), j = 0,... ,N
k
,
(x
1,i
,x
2,−1
),(x
1,i
,x
2,N
k
+1
),i = 0, . .. ,N
h
}.
In [0,T] we introduce the grid {t
n
,n = 0, . .. ,M}
with t
n
= t
n−1
+ ∆t,n = 1,...,M, t
0
= 0,t
M
= T. We
SIMULTECH2013-3rdInternationalConferenceonSimulationandModelingMethodologies,Technologiesand
Applications
692

discretize the integral term of (8) using a rectan-
gular rule and the second order partial derivatives
∂
∂x
(a
∂u
∂x
),
∂
∂y
(b
∂u
∂y
), where a and b are scalar func-
tions, using the usual second order finite difference
operators
∇
∗
h
(a∇
h
u
H
)(x
1,i
,x
2, j
)
=
1
h
a
i+1/2, j
D
−x
1
u
i+1, j
−a
i−1/2, j
D
−x
1
u
i, j
(20)
∇
∗
k
(b∇
k
u
H
)(x
1,i
,x
2, j
)
=
1
k
b
i, j+1/2
D
−x
2
u
i, j+1
−b
i, j−1/2
D
−x
2
u
i, j
(21)
where a
i±1/2, j
= a(x
1,i
±
h
2
,x
2, j
),b
i, j±1/2
=
b(x
1,i
,x
2, j
±
k
2
), D
−x
i
denotes the usual backward
finite difference operator in x
i
direction, i = 1,2.
To compute numerical approximations for u and v in
(x
1,i
,x
2, j
) at time level t
n
, u
n
H
(x
1,i
,x
2, j
), v
n
H
(x
1,i
,x
2, j
),
respectively, we introduce the implicit-explicit finite
difference scheme
D
−t
u
n+1
H
= ∇
∗
h
(d
1
∇
h
u
n+1
H
) + ∇
∗
k
(d
2
∇
k
u
n+1
H
)
+∆t
n
∑
ℓ=0
k
er
(t
n+1
−t
ℓ
)
∇
∗
h
(d
v,1
∇
h
u
ℓ
H
) + ∇
∗
k
(d
v,2
∇
k
u
ℓ
H
)
−β
1
u
n+1
H
+ β
2
v
n
H
in
Ω
H
,
D
−t
v
n+1
H
= (ρ −β
2
)v
n
H
+ β
1
u
n+1
H
in
Ω
H
,
n = 0, . . .,M −1.
(22)
The finite difference method (22) is complemented
with initial conditions
u
0
H
= u
0
, v
0
H
= v
0
in
Ω
H
, (23)
and with the boundary conditions
D
d,η
x
1
u
n
H
(x
1,i
,x
2, j
) +∆t
n
∑
ℓ=0
k
er
(t
n
−t
ℓ
)D
v,η
x
1
u
ℓ
H
(x
1,i
,x
2, j
)
= 0, i = 0,N
h
, j = 0, . . ., N
k
,
D
d,η
x
2
u
n
H
(x
1,i
,x
2, j
) +∆t
n
∑
ℓ=0
k
er
(t
n
−t
ℓ
)D
v,η
x
2
u
ℓ
H
(x
1,i
,x
2, j
)
= 0, i = 0...,N
h
, j = 0,N
k
.
(24)
In (24) the following notations were used
D
d,η
x
1
u
n
H
(x
1,i
,x
2, j
)
=
1
2
d
1,i+1/2, j
D
−x
1
u
n
i+1, j
+ d
1,i−1/2, j
D
−x
1
u
n
i, j
,
D
v,η
x
1
u
ℓ
H
(x
1,i
,x
2, j
)
=
1
2
d
v,1,i+1/2, j
D
−x
1
u
ℓ
i+1, j
+ d
v,1,i−1/2, j
D
−x
1
u
ℓ
i, j
and
D
d,η
x
2
u
n
H
(x
1,i
,x
2, j
)
=
1
2
d
2,i, j+1/2
D
−x
2
u
n
i, j+1
+ d
2,i, j−1/2
D
−x
2
u
n
i, j
,
D
v,η
x
2
u
ℓ
H
(x
1,i
,x
2, j
)
=
1
2
d
v,2,i, j+1/2
D
−x
2
u
ℓ
i, j+1
+ d
v,2,i, j−1/2
D
−x
2
u
ℓ
i, j
.
It can be shown that the truncation error T
H
satis-
fies
kT
ℓ
H
k
∞
= O(h
2
+ k
2
+ ∆t),
provided that
∂
2
u
∂t
2
,
∂
3
u
∂t∂x
2
1
,
∂
3
u
∂t∂x
2
2
,
∂
4
u
∂x
4
1
,
∂
4
u
∂x
4
2
are
bounded in
Ω × [0,T]. As it can be established a
discrete version of the stability inequality (19) for the
errors E
ℓ
u
= u(t
ℓ
) −u
ℓ
H
,E
v
= v(t
ℓ
) −v
ℓ
H
, we conclude
that method (22) is of second order in space and first
order in time.
5 NUMERICAL SIMULATION
In what follows we consider L = 15cm, T = 60days,
ρ = 0.05/day, β
1
= 10
−6
/day, β
2
= 3.6×10
−2
/day,
β = 1, u
0
= 0,v
0
= 10
6
located at the square (7,8) ×
(7,8). The numerical solutions that we present were
obtained with method (22), (23), (24). In Figures 1
and 2 we plot the density of migratory and prolifera-
tion cells defined by by the Fickian model that can be
obtained from the previousmodel considering D
v
= 0.
In this case we took d
1
= d
2
= 0.05cm
2
/day. An in-
creasing of the glioma core is clearly observed.
1
10
2
10
4
10
5
0 5 10 15
0
5
10
15
migration cells, day 5
1
10
5
10
6
0 5 10 15
0
5
10
15
proliferation cells, day 5
Figure 1: Fickian migratory and proliferation profiles at day
5.
The non Fickian migratory and proliferation pro-
files are plotted in Figures 3 and 4 when d
1
= d
2
=
d
v,1
= d
v,2
= 0.025cm
2
/day. We conclude, as ex-
pected, that viscoelastic effect does not change the be-
haviour of the proliferation cells. Moreover, the spa-
tial distribution of such cells presents high gradients.
AViscoelasticModelforGliomaGrowth
693
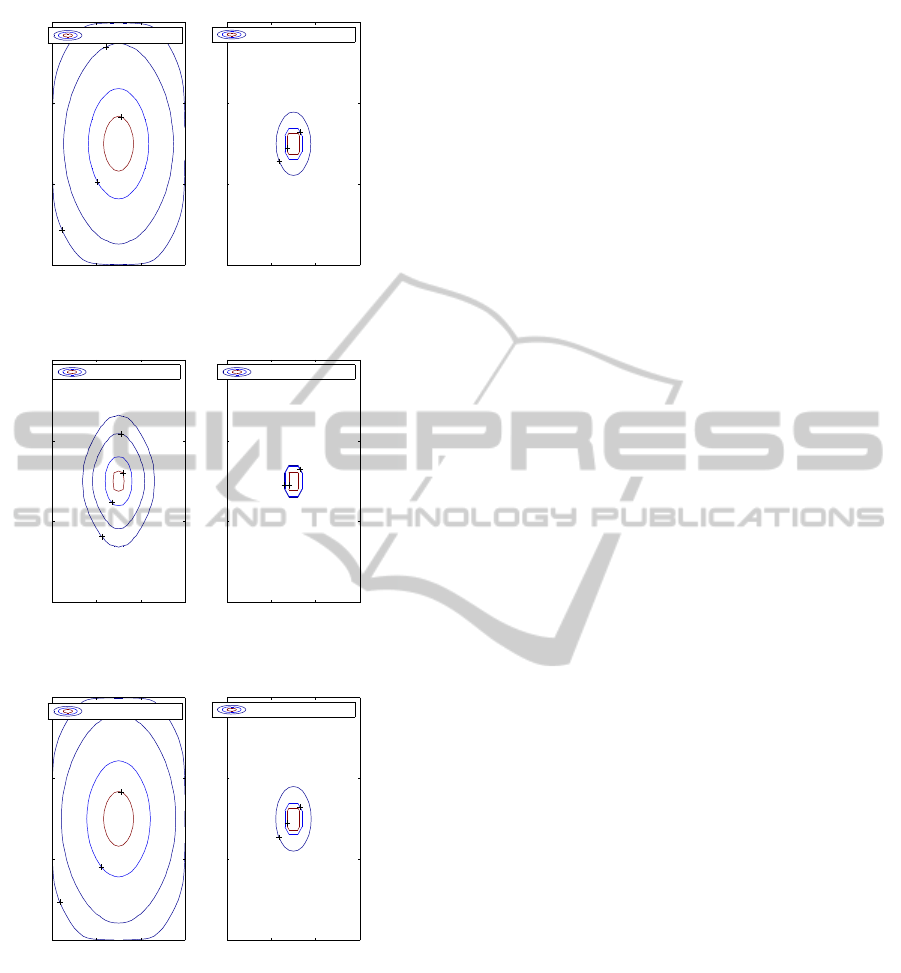
1
10
2
10
4
10
5
0 5 10 15
0
5
10
15
migration cells, day 30
1
10
5
10
6
0 5 10 15
0
5
10
15
proliferation cells, day 30
Figure 2: Fickian migratory and proliferation profiles at day
30.
1
10
2
10
4
10
5
0 5 10 15
0
5
10
15
1
10
5
10
6
0 5 10 15
0
5
10
15
migration cells, day 5 proliferation cells, day 5
Figure 3: Non Fickian migratory and proliferation profiles
at day 5.
1
10
2
10
4
10
5
0 5 10 15
0
5
10
15
migration cells, day 30
1
10
5
10
6
0 5 10 15
0
5
10
15
proliferation cells, day 30
Figure 4: Non Fickian migratory and proliferation profiles
at day 30.
From Figures 2 and 4 we conclude that the non
Fickian migratory cells present higher spreading and
lower concentration than the corresponding cells de-
fined by the Fickian model.
6 CONCLUSIONS
In this paper a mathematical model to describe the
evolution of gliomas cells that take into account the
viscoelastic behaviour of the brain tissue was stud-
ied. Such mathematical model is characterized by an
integro-differential equation of Volterra type that re-
places the diffusion equation usually considered for
the density of migratory cells. This equation was
established assuming that the viscoelastic behaviour
of the brain tissue is described by the Voigt-Kelvin
model. An implicit-explicit numerical method to
compute approximations for migratory and prolifera-
tion densities was presented and some numerical sim-
ulation illustrating the behaviour of the model is in-
cluded. The numerical experiments allow us to con-
clude that the migratory cells defined by the non Fick-
ian model present higher spreading and lower con-
centration than the corresponding cells defined by the
Fickian model. However the behaviour of the prolif-
eration cells seems not be sensitive to the viscoelastic
properties of the brain tissue. In fact the evolution
equation for such cells does not contain a diffusion
part depending on the properties of the surrounding
environment.
ACKNOWLEDGEMENTS
This work was partially supported by the Cen-
tro de de Matem´atica da Universidade de Coim-
bra (CMUC), funded by the European Regional De-
velopment Fund through the program COMPETE
and by the Portuguese Government through the
FCT - Fundac¸˜ao para a Ciˆencia e Tecnologia un-
der the projects PEst-C/MAT/UI0324/2011and by the
project UTAustin/MAT/0066/2008.
REFERENCES
(2002). Mathematical Biology- An Introduction. Springer
Verlag.
Edward, D. and Cohen, D. (1995). A mathematical model
for a dissolving polymer. AIChE Journal, 41:2345–
2355.
Edwards, D. (1996). Non-fickian diffusion in thin polymer
films. Journal of Polymer Science, Part B: Polymer
Physics Edition, 34:981–997.
Edwards, D. A. (2001). A spatially nonlocal model
for polymer-penetrant diffusion. Journal Zeitschrift
f¨ur Angewandte Mathematik und Physik (ZAMP),
52:254–288.
Edwards, D. A. and Cohen, D. S. (1995). An unusual mov-
ing boundary condition arising in anomalous diffusion
problems. SIAM Journal on Applied Mathematics,
55:662–676.
Fedotov, S. (1998). Traveling waves in a reaction-diffusion
system: diffusion with finite velocity and kolmogorov
SIMULTECH2013-3rdInternationalConferenceonSimulationandModelingMethodologies,Technologiesand
Applications
694

- petrovskii - piskunov kinetics. Pysical Review E,
58:5143–5145.
Fedotov, S. (1999). Nonuniform reaction rate distribution
for the generalized fisher equation: Ignition ahead of
the reaction front. Pysical Review E, 60:4958–4961.
Fedotov, S. and Iomin, A. (2007). Migration and prolifer-
ation dichotomy in tumor-cell invasion. Physical Re-
view Letters, 77:1031911(1)–(10).
Fedotov, S. and Iomin, A. (2008). Probabilistic approach to
a proliferation and migration dichotomy in tumor cell
invasion. Physical Review E, 77:1031911(1)–(10).
G.Franceschini (2006). The mechanics of humain brain tis-
sue. PhD thesis, University of Trento.
Giese, A., Kluwe, L., Laube, B., Meissner, H., Berens, M.,
and Westphal, M. (1996). Migration of human glioma
cells on myelin. Neurosurgery, 38:755–764.
Habib, S., Molina-Par´ıs, C., and Deisboeck, T. (2003).
Complex dynamics of tumors: modeling an energing
brain tumor system with coupled reaction-diffusion
equations. Physica A, 327:501–524.
Harpold, H., Jr, E. A., and Swanson, K. (2007). The evolu-
tion of mathematical modeling of glioma proliferation
and invasion. Journal of Neuropathology and Experi-
mental Neurology, 66:1–9.
Hassanizadeh, S. (1996). On the transient non fickian dis-
persion theory. Transport in Porous Media, 23:107–
124.
Humphrey, J. (2003). Continuum biomechanics of soft bio-
logical tissues. Proceedings of RoyalSociety London,
459:3–46.
Joseph, D. and Preziosi, L. (1989). Heat waves. Review of
Modern Physics, 61:47–71.
Mehrabian, A. and Abousleiman, Y. (2011). A general so-
lution to poroviscoelastic model of hydrocephalic hu-
main brain tissue. Journal of Theoretical Biology,
29:105–118.
Neuman, S. P. and Tartakovsky, D. M. (2009). Perspective
on theories of anomalous transport in heterogeneous
media. Advances in Water Resources, 32:670–680.
Rockne, R., Jr, E. A., Rockhill, J., and Swanson, K. (2009).
A mathematical model for brain tumor response to
radiation therapy. Journal of Mathematical Biology,
58:561–578.
Shaw, S. and Whiteman, J. R. (1998). Some partial differ-
ential volterra equation problems arising in viscoelas-
ticity. In Proceeding of the Conference on Differential
Equations and their Applications, Brno, August 2529,
1997, ed. R. P. Agarwal, F. Neuman, J.Vosmansky.
Swanson, K., Alvord, E., and Murray, J. (2000). A quantita-
tive model for differential motility of gliomas in grey
and white matter. Cell Proliferation, 33:317–330.
Swanson, K., Bridge, C., Murray, J., and Alvord, E. (2003).
Virtual and real brain tumors: using mathematical
modelig to quantify glioma growth and invasion. Jour-
nal of the Neurological Sciences, 216:17–31.
Tracqui, P., Cruywagen, G., Woodward, D., Bartoo, G.,
Murray, J., and Jr, E. A. (1995). A mathematical
model of glioma growth: the effect of chemotherapy
on spatio-temporal growth. Cell Proliferation, 28:17–
31.
AViscoelasticModelforGliomaGrowth
695
