
Fully Organic Graphene Oxide-based Sensor with Integrated Pump
for Sodium Detection
Jingfeng Huang
1,2,3
, James Harvey
1,2,3
, Hu Chen
1,2,3
, Steve Faulkner
3
, James King
3
Myra A. Nimmo
2,3
and Alfred Tok I. Y.
1,2
1
School of Materials Science and Engineering, Nanyang Technological University,
50 Nanyang Avenue, Singapore, 637553, Singapore
2
Institute for Sports Research, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798, Singapore
3
School of Sport, Exercise and Health Sciences, Loughborough University,
Ashby Road, Loughborough, Leicestershire, LE11 3TU, U.K.
Keywords: Graphene Oxide, Real-time, Sweat, Organic Sensor, Sodium Sensor, Biosensor, Field Effect Transistor.
Abstract: Sweat is produced by the body nat.urally during physical activity and this fluid can be analysed in real-time
to reflect the body’s hydration and electrolyte status. This paper reports a new type of organic disposable
sensor pump that integrates sweat collection and sodium (Na
+
) ion sensing into cotton threads. This
integration allows the sensor platform to be small, portable and wearable; thus allowing potential advantage
to interface with the human body during field exercises enabling the provision of real-time data for
immediate intervention. The sensor uses a sodium-selective Ion Selective Electrode (ISE) modified
graphene oxide transducer intertwined with a thread pump. In this paper, we present the characterisation,
synthesis and sensing data of this sensor.
1 INTRODUCTION
During exercise, sweating can lead to the loss of
electrolytes such as sodium and potassium (Baker et
al., 2009). With increasing sweat rate, only Na
+
and
Cl
-
(from extracellular compartments)
concentrations in sweat tend to increase while Ca
2+
(from intracellular space) decreases; K
+
and Mg
2+
(from intracellular space) remain unchanged
(Costill, 1977). Electrolyte loss through urine during
exercise is small because of decreased urine
formation and increased renal Na
+
reabsorption.
Deficits of 5% to 7% of the body’s Na
+
and Cl
-
ions
can be lost through sweat in comparison to less than
1.2% loss for K
+
and Mg
2+
. (Costill, 1977).
Measurement of sodium and potassium ions in sweat
can be used to predict changes in serum (Baker et
al., 2009). Thus sodium levels in sweat can be also
used to predict exercise-associated hyponatremia.
Therefore, sodium measurement in sweat is
important and essential. In this paper, we present the
characterisation, synthesis and sensing data of a
novel disposable, organic, low-cost, graphene-based
and pump-integrated sodium sensor suitable for real-
time sensing of sweat sodium concentration in field
conditions.
2 RESULTS AND DISCUSSION
2.1 Idea of Fully Organic Sodium
Sensor
From a list of currently available sodium portable
sensors (Table 1), there is a niche to develop a
disposable and low-cost sodium sensor suitable for
real-time sensing in field conditions.
There is a demand for such a sensor platform in
the fast-growing biosensor market which is
projected to reach US$12 billion by 2015 propelled
by the growing population and health issues (GIA,
2012).
Increasingly graphene is the transducer material
of choice because of its ability to detect single
molecule binding events (Schedin et al., 2007). This
is due to its exceptional low-noise electronic
material property. Graphene, being a 2-dimensional
material with high surface area to volume ratio, is
also very sensitive to small perturbations on its
surface and these perturbations change its electrical
83
Huang J., Harvey J., Chen H., Faulkner S., King J., A. Nimmo M. and Tok I. Y. A..
Fully Organic Graphene Oxide-based Sensor with Integrated Pump for Sodium Detection.
DOI: 10.5220/0004636200830088
In Proceedings of the International Congress on Sports Science Research and Technology Support (icSPORTS-2013), pages 83-88
ISBN: 978-989-8565-79-2
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

Table 1: Comparison of practical and portable sodium sensors.
Na
+
Sensors Minimum
Sample
Volume
Useful
Range
Precision Size Disadvantage
Wescor brand –Sweat
Check Conductivity
Meter (to be used with
Macroduct system)
8μl 0-150mM
2% from
75-110mM
10x20x16 cm;
1kg
-Non-real time
-Not suitable for field testing
Wescor brand –
Neonatal Sweat
Analysis system
Needed but
not indicated
3-200mM
<1% from
25-150mM
19x13x5 cm;
0.5kg
-Not suitable for field test
Centre for sensor web
technologies – Wearable
sodium sensor
(Schazmann et al.,
2010)
Needed but
not indicated
0mM-
100mM
3% RSD in
lab testing
Not stated
-Requires special plastic molds
- Requirement to store electrode
in special liquids when not in use
-Not disposable, bacteria growth
observed
BIOTEX—Biosensing
Textiles for Personalised
Healthcare Management
(Coyle et al., 2010)
Needed but
not indicated
Not stated Not stated Not stated
-Requirement to store electrode in
special liquids when not in use.
-Not real-time as contact between
fabric and electrode is poor
resistivity drastically (Huang et al., 2013b).
Briefly, graphene is a single atom thick film
made up of carbon atoms connected in a sp
2
hybridized network. It has novel properties such as
size tunable band gap and high carrier mobility.
Graphene has already demonstrated that it is able to
detect single gas molecules (Schedin et al., 2007).
Graphene can be obtained via several methods,
including chemical vapour deposition (Huang et al.,
2013c), mechanical exfoliation (Novoselov et al.,
2004) and chemical methods (Larisika et al., 2012).
However, the chemical method has several
advantages: low-cost, scalability and aqueous
processability. Using graphene from the chemical
route can lower the cost of sensors down to less than
£1 per chip. Graphene produced via the chemical
route contains several oxygen groups even after
reduction and is thus sometimes termed reduced
graphene oxide or reduced aqueous graphene
(Huang et al., 2013d). In later discussions, we term
the product as reduced graphene oxide (RGO).
RGO-based bio-sensors have also been reported to
be able to detect single bacteria (Mohanty and Berry,
2008), cell-activities (He et al., 2010) and label-free
DNA (Ohno et al., 2009). The graphene transducer
can be used in an optical, amperometrical or
acoustical sensor setup.
This paper uses the amperometric field effect
transistor detection setup (Figure 1) as it can
eliminate the need for expensive, bulky, highly-
specialised equipment in diagnostic screening tests.
For selectivity, any receptors (e.g. sodium
ionophore) can be attached to the amperometric
graphene transducer to enable detection of a large
variety of biological molecules with biomedical
significance in real time.
Figure 1: Schematic of amperometric field effect transistor
(FET) biosensor setup.
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
84

Figure 2: Field emission scanning electron microscopy images of (a) graphene oxide flakes on silicon dioxide wafer (b)
cotton thread after plasma treatment (c) graphene oxide on cotton thread taken under similar magnification.
2.2 Integrating Sweat Collection
Methods for the collection of sweat include whole
body washdown (WBW) believed to be the most
accurate and reliable with analysis undertaken later.
However this method is limited to cycling and
requires a controlled laboratory setting.
Alternatively, regional skin collection is relatively
simple, practical for field studies and is able to
accurately and reliably predict WBW sodium
concentrations (Patterson et al., 2000). Regional skin
collection methods with occlusive coverings have
been reported to result in falsely high electrolyte
readings because of electrolyte leeching from the
stratum corneum of the skin (Weschler, 2008).
However, if the skin can be kept dry by a sweat
wicking material, it could be possible to obtain
reliable estimates of local sweat electrolyte
concentrations. A wicking sensor can thus prevent
reabsorption of Na
+
back into the body. It will then
be possible to measure the true Na
+
excretion. After
these considerations, we designed a novel RGO-
based, amperometric sensor platform with an
integrated pump which can be used for real-time
sensitive detection of sodium ions.
2.3 Fabrication and Characterisations
Special large Graphene Oxide (GO) flakes, a
precursor to RGO, were prepared by modified
Hummer’s method from natural graphite flakes (3-
5mm). Briefly, 2g of graphite flakes were mixed
with H
2
SO
4
(12ml) and stirred for 5 hours at 80˚C.
The mixture was ultra-sonicated for 1 hour and
diluted with DI water (500ml). The suspension was
then filtered using 0.2μm filter to obtain dry pre-
oxidized graphite powders. To fully oxidize the
graphite, H
2
SO
4
(120ml) and KMnO
4
(15g) was
added to the powder and stirred for 2 hours before
diluting with 950ml of DI water slowly in ice-bath
and finally stopping the reaction using H
2
O
2
(20ml).
The upper portion of the solution was collected,
filtered and washed to remove remaining metal ions
and acid. This forms the stock GO solution
containing large GO flakes used in subsequent
experiment. To image the large GO flakes, GO
solution was drop-cast on silicon dioxide flat
substrate and scanning electron microcopy was used
(Figure 2a). Using this method, the average size of
the GO flakes are 700 μm
2
and this is 7 orders of
magnitude larger (Larisika et al., 2012) than existing
literatures. Larger GO flakes exhibit remarkable
lower sheet resistance, lower intra-flake resistance
and higher hole carrier mobility which could result
in a more sensitive sensor.
To prepare the cotton substrate for the RGO,
cotton threads were treated with oxygen (25%)
plasma. This treatment removes natural wax and
increases oxygen moieties on the surface of the
thread, making subsequent chemical
functionalization possible. After a 50 minute plasma
treatment, the structure of the cotton thread is still
intact (Figure 2b). Then (3-Aminopropyl)
triethoxysilane (2% v/v ethanol) was used to
functionalise the cotton surface to make it positively
charged. The functionalised thread was then
submerged in the GO stock solution for 30 minutes.
As the surface of GO is negatively charged, during
the incubation period, the GO will be
electrostatically attached onto the thread.
It had been reported that GO can also be coated
directly onto the cotton thread using a drip-and-dry
method (Shateri-Khalilabad and Yazdanshenas,
2013). However, attaching the graphene using the
electrostatic attraction method allows self-assembly
and termination, thus allowing controlled 1-2 layers
of GO to be adhered to the thread (Figure 2c). The
2-dimensional material characteristics of GO is
preserved with 1-2 layers of GO as the hole or
electrons carriers can only move along the planar
direction. Having more than two layers of GO will
allow the carriers to travel in parallel (3-
FullyOrganicGrapheneOxide-basedSensorwithIntegratedPumpforSodiumDetection
85

dimensional) and decreases the sensor’s sensitivity
to any perturbations from the ionophore.
After the attachment of GO to the thread, a
chemical reduction is needed to remove oxygen
moieties from the carbon backbone of GO and
restore its electrical conductivity. The thread is
placed in a sealed petri dish with 500μl of hydrazine
monohydrate and left overnight at 70˚C. The
hydrazine vapor produced will reduce the GO into
RGO. This RGO-lined cotton thread forms the RGO
transducer. The exposed RGO transducer is sensitive
to all molecules, such as H
2
O (humidity) and NO
2
(gas) molecules. Therefore a coat is needed to
protect the RGO transducer from the environment.
To achieve selectivity of sodium on the RGO
surface, a sodium ionophore cocktail was coated
onto the RGO transducer. Briefly, bis(1-butylpentyl)
adipate, sodium ionophore (71733) and poly(vinyl
chloride) high molecular weight, (Sigma-Aldrich, St
Louis, USA), were dissolved in tetrahydrofuran
solvent and then the ionophore cocktail was left
overnight to stabilise before coating on the RGO
transducer. The ionophore used is an electrically
neutral, lipophilic ion-complexing agent of small
relative molar mass. Two coatings of sodium
ionophore were applied to the RGO transducer to
ensure complete coverage.
The ionophore-covered RGO transducer was
then fixed across a polyethylene terephthalate (PET)
platform and attached to a multimeter (Agilent
U1273A and E3ABAG) via copper wire and clips.
Another plasma-treated thread was coiled around the
Figure 3: The graphene oxide based sensor with integrated
fabric pump. (For interpretation of the colored dyes on the
figure, kindly refer to the online version of this article).
RGO transducer. This allows the liquid analyte to
flow through it to reach the transducer (figure 3).
One of the thread ends is attached to a silicone
rubber liquid analyte inlet and the other to a cotton
wool excess liquid analyte reservoir. The large
collection reservoir allows constant flow of liquid
analyte through the thread. This thread acts as a
pump using capillary action and no external power
source is needed (Reches et al., 2010; Li et al.,
2009). After the sensing setup is completed, colored
dyes are used to detect the movement of liquid
analyte.
2.4 Pump Testing and Sodium Sensing
From Figure 3, it can be observed that the blue and
then red colored dyes travel cleanly along the fabric
pump. The wicking rate increases exponentially with
increasing plasma treatment time on the cotton
thread ca. 3mm/min, 10mm/min and 50mm/min
after 10, 30, 50 minutes of oxygen plasma treatment
respectively. In the experiments, 50 minutes of
plasma treatment on all cotton thread was used.
Then the colored dyes were replaced with sodium
analyte for sensing data collection.
To test the sodium detection window of the
sensor, different concentrations of sodium chloride
solution were added to fill the analyte inlet and
allowed to flow through the thread pump. The liquid
analyte travels via capillary action along the thread
pump towards the ionophore-coated transducer. The
ionophores selectively extract sodium ions into the
hydrophobic membrane phase and transport these
ions across the barrier by carrier translocation. The
ions carried across the ionophore will then cause
perturbations on the RGO surface and change the
electrical resistivity. This change in electrical
property can be detected by a multimeter and the
data is then sent to a data-logger. A sensing window
is thus obtained and presented in Figure 4.
As the fabricated RGO transducer is a p-type
semiconductor (Huang et al., 2013a), when positive
sodium ions travel through the ionophore onto the
surface, it induces a positive gating effect (Huang et
al., 2013b) and increases the RGO’s electrical
resistance. The electrical resistance changes
according to the change in the concentrate on of
sodium chloride.
As the concentration of the sodium chloride
increased from 0mM to 90mM, the electrical
resistance through the RGO transducer increased
from 615.2 to 638.5kΩ correspondingly. A 90mM
sodium ion concentration contributed a 3.9%
increase in electrical resistance across the RGO
transducer.
Patterson et al (Patterson et al., 2000) reported
whole-body sweat Na
+
concentrations of 24.1±15.0
mM (mean ± SD). Na
+
levels at specific regions
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
86
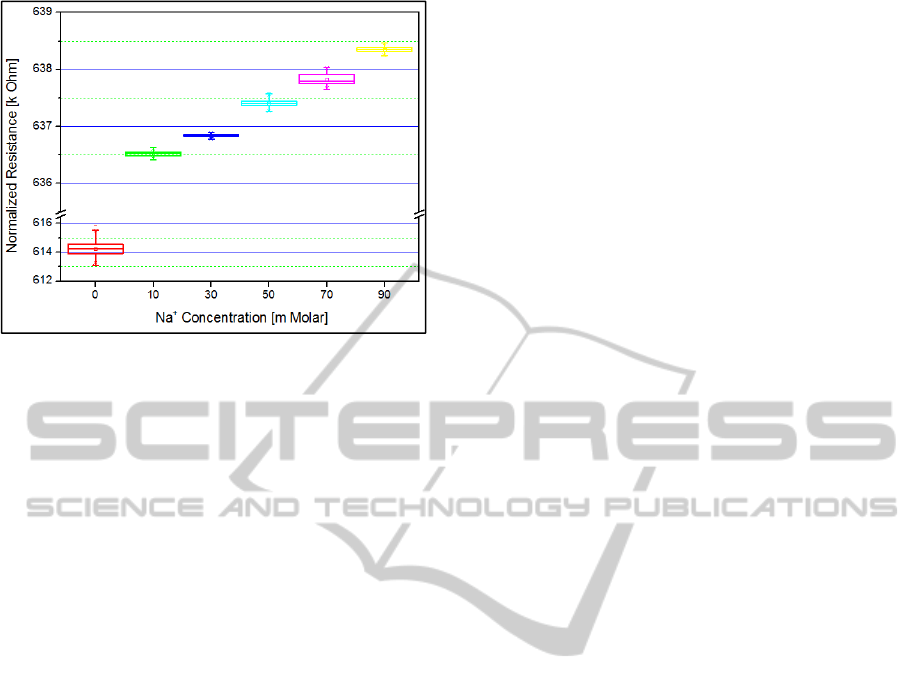
Figure 4: Normalized electrical resistance output vs.
sodium ion concentration input.
(such as the forearm, chest and scapula) were
consistently higher (mean Na+ concentration at each
site: 42.2±25.8, 47.6±25.7 and 42.2±24.8 mM
respectively). Therefore, our sensor operates within
the typical physiological range. As can be seen from
the large standard deviations for sweat Na
+
levels,
sweat composition can vary widely between
individuals. Our sensor is therefore of interest in the
field of exercise science: real-time monitoring of
sweat Na
+
losses allows for the development of
athlete-specific rehydration strategies.
In theory, the lower detection window of the
reported transducer is only limited to the sodium
ionophore applied and electronic amplification
equipment used, as graphene had already been
shown to detect 1 single molecular binding events
(Schedin et al., 2007). As a comparison, a single liter
of 10mM of sodium chloride solution contains
6.023x10
26
number of sodium ions. With such high
sensitivity, this RGO-based sensor can also be
modified with antibodies or other ionophores to
detect other specific ions or biomolecules of interest
to the community. The presented sodium sensor
could potentially be used to diagnose dehydration in
the ageing population and used in the study of the
biology of sweat in older people. Future work on the
disposable and low-cost real-time fully-organic
sensor platform would include optimizing the
fabrication technique for mass-production and
sensing for other ions or proteins.
3 CONCLUSIONS
During exercise, one of the body’s major ionic
deficits of concern is Na
+
. Measurements of sodium
ions in sweat had been reported previously to predict
changes in serum. In this paper, we have shown the
fabrication, characterisation and physiologically-
relevant sodium-ions detection limits of a novel
RGO-based sensor with an integrated pump that is
fully organic, low-cost and disposable. As the sensor
is small, light and wearable, it has enormous
potential to be integrated onto the human body
during field training to obtain real-time data for
immediate intervention.
ACKNOWLEDGEMENTS
The research was supported by the National Institute
for Health Research (NIHR) Diet, Lifestyle &
Physical Activity Biomedical Research Unit based at
University Hospitals of Leicester and Loughborough
University. The views expressed are those of the
authors and not necessarily those of the NHS, the
NIHR or the Department of Health. The research
was also funded by the Institute for Sports Research
(ISR) of Nanyang Technological University (NTU).
REFERENCES
Baker, L. B., Stofan, J. R., Hamilton, A. A. & Horswill, C.
A., 2009. Comparison of regional patch collection vs.
whole body washdown for measuring sweat sodium
and potassium loss during exercise. Journal of Applied
Physiology, 107, 887-895.
Costill, D. L., 1977. Sweating: Its Composition and
Effects in Body Fluids. Annals of the New York
Academy of Sciences, 301, 160-174.
Coyle, S., King-Tong, L., Moyna, N., O'gorman, D.,
Diamond, D., Di Francesco, F., Costanzo, D., Salvo,
P., Trivella, M. G., De Rossi, D. E., Taccini, N.,
Paradiso, R., Porchet, J. A., Ridolfi, A., Luprano, J.,
Chuzel, C., Lanier, T., Revol-Cavalier, F.,
Schoumacker, S., Mourier, V., Chartier, I., Convert,
R., De-Moncuit, H. & Bini, C., 2010. Biosensing
Textiles for Personalised Healthcare Management.
Information Technology in Biomedicine, IEEE
Transactions on, 14, 364-370.
Gia, 2012. Biosensors in Medical Diagnostics. Global
strategic business report. CA.
He, Q., Sudibya, H. G., Yin, Z., Wu, S., Li, H., Boey, F.,
Huang, W., Chen, P. & Zhang, H., 2010. Centimeter-
Long and Large-Scale Micropatterns of Reduced
Graphene Oxide Films: Fabrication and Sensing
Applications. ACS Nano, 4, 3201-3208.
Huang, J., Harvey, J., Chen, H., Fam, W. H. D., Nimmo,
M. A. & Tok, I. Y. A., 2013a. Growth of Graphene
Oxide Using Ethanol CVD. Procedia Engineering.
Huang, J., Harvey, J., Fam, W. H. D., Nimmo, M. A. &
FullyOrganicGrapheneOxide-basedSensorwithIntegratedPumpforSodiumDetection
87

Tok, I. Y. A., 2013b. Novel Biosensor for Interleukin-
6 Detection. Procedia Engineering.
Huang, J., Larisika, M., Fam, W. H. D., He, Q., Nimmo,
M. A., Nowak, C. & Tok, A. I. Y., 2013c. The
extended growth of graphene oxide flakes using
ethanol CVD. Nanoscale, 5, 2945-2951.
Huang, J., Larisika, M., Nowak, C. & Tok, I. Y. A.,
2013d. New Methods in Aqueous Graphene (Graphene
Oxide) Synthesis for Biosensor Devices.
Larisika, M., Huang, J., Tok, A., Knoll, W. & Nowak, C.,
2012. An improved synthesis route to graphene for
molecular sensor applications. Materials Chemistry
and Physics, 136, 304-308.
Li, X., Tian, J. & Shen, W., 2009. Thread as a Versatile
Material for Low-Cost Microfluidic Diagnostics. ACS
Applied Materials & Interfaces, 2, 1-6.
Mohanty, N. & Berry, V., 2008. Graphene-Based Single-
Bacterium Resolution Biodevice and DNA Transistor:
Interfacing Graphene Derivatives with Nanoscale and
Microscale Biocomponents. Nano Letters, 8, 4469-
4476.
Novoselov, K. S., Geim, A. K., Morozov, S. V., Jiang, D.,
Zhang, Y., Dubonos, S. V., Grigorieva, I. V. & Firsov,
A. A., 2004. Electric Field Effect in Atomically Thin
Carbon Films. Science, 306, 666-669.
Ohno, Y., Maehashi, K., Yamashiro, Y. & Matsumoto, K.,
2009. Electrolyte-Gated Graphene Field-Effect
Transistors for Detecting pH and Protein Adsorption.
Nano Letters, 9, 3318-3322.
Patterson, M. J., Galloway, S. D. R. & Nimmo, M. A.,
2000. Variations in Regional Sweat Composition in
Normal Human Males. Experimental Physiology, 85,
869-875.
Reches, M., Mirica, K. A., Dasgupta, R., Dickey, M. D.,
Butte, M. J. & Whitesides, G. M., 2010. Thread as a
Matrix for Biomedical Assays. ACS Applied Materials
& Interfaces, 2, 1722-1728.
Schazmann, B., Morris, D., Slater, C., Beirne, S., Fay, C.,
Reuveny, R., Moyna, N. & Diamond, D., 2010. A
wearable electrochemical sensor for the real-time
measurement of sweat sodium concentration.
Analytical Methods, 2, 342-348.
Schedin, F., Geim, A. K., Morozov, S. V., Hill, E. W.,
Blake, P., Katsnelson, M. I. & Novoselov, K. S., 2007.
Detection of individual gas molecules adsorbed on
graphene. Nat Mater, 6, 652-655.
Shateri-Khalilabad, M. & Yazdanshenas, M. E., 2013.
Fabricating electroconductive cotton textiles using
graphene. Carbohydrate Polymers, 96, 190-195.
Weschler, L. B., 2008. Sweat electrolyte concentrations
obtained from within occlusive coverings are falsely
high because sweat itself leaches skin electrolytes.
Journal of Applied Physiology, 105, 1376-1377.
icSPORTS2013-InternationalCongressonSportsScienceResearchandTechnologySupport
88
