
Clinical, Functional and Kinematic Correlations using the Virtual
Reality System Toyra® as Upper Limb Rehabilitation Tool in People
with Spinal Cord Injury
Iris Dimbwadyo-Terrer
1
, Fernando Trincado-Alonso
1
, Ana de los Reyes-Guzmán
1
,
Alberto Bernal-Sahún
2
, Patricia López-Monteagudo
2
, Begoña Polonio-López
3
and Ángel Gil-Agudo
1
1
Biomechanics and Technical Aids Department, National Hospital for Spinal Cord Injury,
Finca la Peraleda s/n, Toledo, Spain
2
Indra Systems, Madrid, Spain
3
University of Castilla la Mancha, Talavera de la Reina, Spain
Keywords: Upper Limb, Rehabilitation, Spinal Cord Injury, Toyra, Virtual Reality.
Abstract: The aim of this study was to prove the validity and efficacy of the Virtual Reality (VR) System Toyra® as
an assessment and rehabilitation tool for people with tetraplegia. We analysed the correlation between
clinical and functional parameters with kinematic variables of upper limbs during a training protocol using
Toyra®. Eighteen patients with cervical spinal cord injury (SCI) were selected to perform the study by
comparing 2 treatments: patients in an intervention group (IG) conducted a program that included 12
sessions with Toyra® Activities of Daily Living (ADLs) module for 3 weeks, while a control group (CG)
only had the traditional rehabilitation. Kinematic variables (shoulder, elbow and hand joint range of motion)
were correlated to clinical [Motor Index (MI), Muscle Balance (MB)] and functional [Functional
Independence Measure (FIM), Spinal Cord Independence Measure II (SCIM II), Barthel Index (BI)]
evaluation scores. The results of the study showed a high correlation between these variables and also
statistically significant differences (p=0.039) in a kinematic parameter (wrist extension), after treatment and
in the follow-up evaluation. Toyra® system has been validated as upper limb assess and rehabilitation tool
in people with SCI, to measure the patient´s functional evolution and improve the movement in upper limbs.
1 INTRODUCTION
The worldwide estimate of the prevalence of spinal
cord injury (SCI) is 223-755 per million people, with
an incidence of 10.4-83 per million individuals per
year
(Wyndaele and Wyndaelem, 2006). Fifty
percent of the patients with SCI are diagnosed as
complete, with one-third of them reported as
tetraplegic.
In tetraplegia, the arm and hand function is
affected to varying degrees, depending on the level
and severity of the injury (Harvey et al., 2001).
Studies have shown that one of the greatest needs
of patients with tetraplegia is the improvement in
upper limb function
(Snoek et al., 2004).
In this respect, therapy aimed at upper
extremities in people with tetraplegia is of
paramount importance.
Considerable efforts have been directed towards
the development of new upper limb (UL) function
rehabilitation therapies using robots, virtual reality
(VR), passive workstations (passive antigravity
orthosis), and functional electrical stimulation (FES)
systems
(Oess et al., 2012).
Specifically, in an effort to promote task oriented
and repetitive movement training of motor skills the
use of VR with simulated environments has emerged
as a useful tool
(Stewart et al., 2007).
Using VR, users are able to interact with images,
manipulate virtual objects, and perform other actions
in a way that allows them to “immerse” themselves
within the simulated environment and thereby create
a feeling of “presence” in the virtual world
(Weiss et
al., 2006). In comparison with conventional
rehabilitation, VR technology increases the range of
possible tasks, while partly automating and
quantifying therapy procedures, and improving
patient motivation using real-time task evaluation
and reward
(Eng et al., 2007).
81
Dimbwadyo-Terrer I., Trincado-Alonso F., de los Reyes-Guzmán A., Bernal-Sahún A., López-Monteagudo P., Polonio-López B. and Gil-Agudo Á..
Clinical, Functional and Kinematic Correlations using the Virtual Reality System Toyra
R
as Upper Limb Rehabilitation Tool in People with Spinal Cord
Injury.
DOI: 10.5220/0004642600810088
In Proceedings of the International Congress on Neurotechnology, Electronics and Informatics (VirtRehab-2013), pages 81-88
ISBN: 978-989-8565-80-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

To measure the effectiveness of such techniques,
an evaluation, using clinical and functional scales, is
performed before and after the treatment program to
identify motor and functional recovery. In evaluation
studies of upper extremity function in people with
tetraplegia, a functional test supplemented with a
test in which the subject is asked to perform several
activities of daily living (ADL) are used
(Van Tuijl
et al., 2002). Two of the most commonly used
functional evaluations, for patients with tetraplegia,
are the Functional Independence Measure (FIM) and
the Spinal Cord Independence Measure II (SCIM II).
There tests are valid and reliable, and show strong
correlation with each other.
However, a better understanding of human
movement requires more objective testing and
accurate analysis of motion, to accurately describe
the arm movements during functional activities.
Kinematic analysis is one method that can provide
this understanding
(Alt Murphy et al., 2006).
The study carried out by Cacho et al. (2011)
showed correlation between some kinematic
variables and clinical measures, in people with SCI,
during the execution of ADLs
(Cacho et al., 2011).
The objective of the current study is to analyse
the correlation between clinical and functional
assessments and the kinematic variables of UL. This
is performed by comparing the results from a
treatment based on VR with those from a
conventional rehabilitation treatment in patients with
complete tetraplegia.
2. MATERIAL AND METHODS
2.1 Participants
Twelve intervention subjects (4 females and 8
males; aged 33.58±14.11 years, 3.67±1.78 months
after injury) and 6 control subjects (3 females and 3
males, aged 42±13.56 years, 6.67±2.16 months after
injury) participated in the study. The subjects’
demographic and clinical characteristics are showed
in the Table 1.
Eligible participants met the following criteria:
(1) at least 18 years of age; (2) less than 12 months
from the injury; (3) complete spinal cord injury
according to the ASIA´s impairment scale at the
level of C5 to C8 (A-B ASIA level); (4) no history
of traumatic or cognitive pathology that can affect
the UL movements; (5) normal or corrected-to-
normal vision and hearing; (6) no history of
technology addiction; and (7) no history of epilepsy
and pregnancy. Each subject gave informed consent
voluntarily which was approved by our local Ethics
Committee.
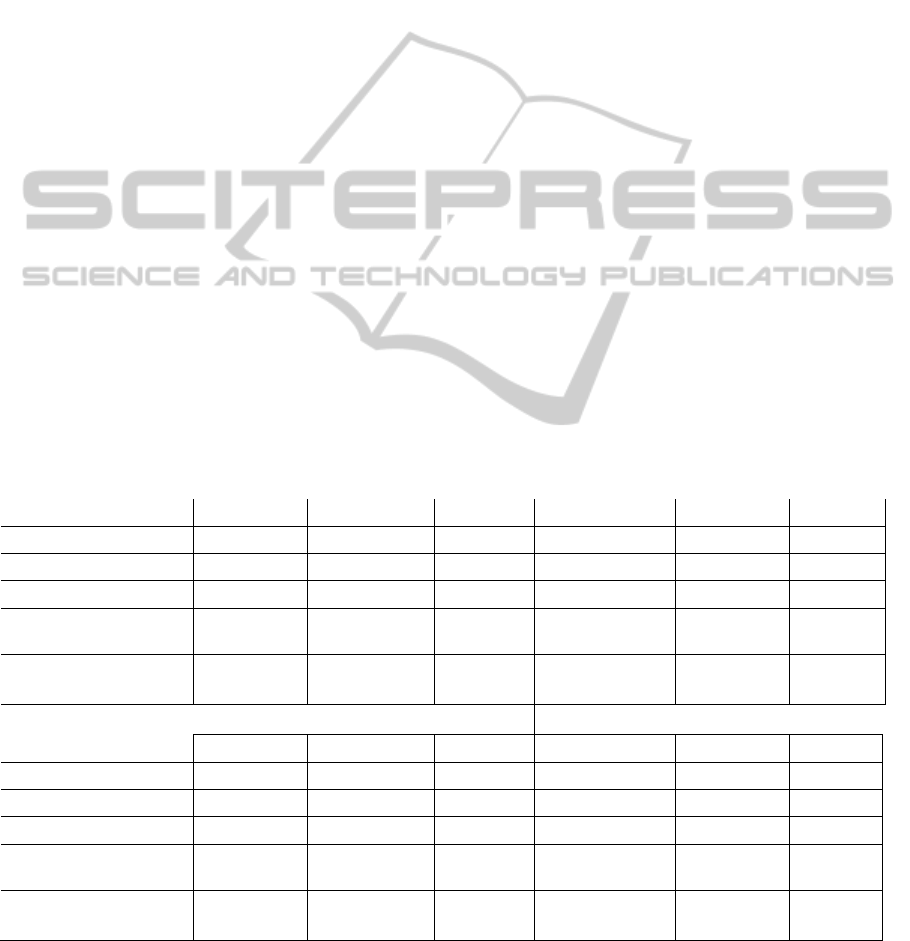
Table 1: Subjects´ demographic and clinical characteristics
(mean and standard deviation).
Control Group
(n=6)
Intervention Group
(n=12)
Gender
(female/male)
3/3 4/8
Age [years]
42±13.56 33.58±14.11
Dominance
(right/left)
3/3 5/7
Level of injury
(C5-C8)
C5 (4), C6 (1) ,
C7 (1)
C5 (5), C6 (3), C7 (3),
C8 (1)
ASIA (A-D)
A(3),B(3) A(8), B(4)
Time since injury
[months]
6.67±2.16 3.67±1.78
Etiology of damage
(traumatic/postsurgi
cal/vascular)
6/0/0 11/1/0
2.2 Experimental Design
This is a research study comparing 2 treatments.
Patients in intervention group (IG) took part in a
treatment program that included 12 sessions with
Toyra® ADLs module using 3 levels of difficulty
for 3 weeks. Simultaneously to Toyra® treatment,
patients also received a daily session of conventional
Occupational Therapy and Physiotherapy. Patients
assigned to the control group (CG) only had the
conventional treatment without receiving the
described Toyra® sessions.
Figure 1: Experimental Design. The flowchart represents
the experimental design followed during the study.
NEUROTECHNIX2013-InternationalCongressonNeurotechnology,ElectronicsandInformatics
82

Every subject (CG and IG) was evaluated twice:
at the beginning of the study and at the end, using
both the VR system and the clinical and functional
scales. A small sample from each group was
followed up and assessed 3 months after the study,
to measure if there were kinematic, functional or
clinical changes during this period (Figure 1).
In order to prove the validity of the VR system
Toyra® as an assessing tool, we correlated clinical
and functional evaluations with kinematic variables
of UL movements in patients with tetraplegia at
three different points: before and after the treatment,
and 3 months later (follow-up).
2.3 Treatment
The treatment system used was the VR Toyra®,
which was comprised of motion capture elements
that reproduce, in real time, the movements of the
patient through an avatar displayed on an LCD
screen, the characteristics of the system having been
described previously
(Gil-Agudo et al., 2012). A
series of objects are shown, and the avatar, which
represents the patient, has to touch them, while
following predefined treatment goals.
In the current study we have conducted one type
of interactive therapy session with the Toyra®
system:
- Activities of Daily Living (ADLs) Session: The
main objective is to achieve the maximum degree of
autonomy that is possible while performing ADLs
training in the VR system. In this session the
monitor displayed several objects (spoon, fork,
comb, sponge), asking the patient to reproduce the
movements necessary to perform the corresponding
ADL activities (eating with spoon, eat with a fork,
combing hair and wash your face with a sponge).
2.4 Assessment
For the kinematic capture process we used a motion
capture system based on inertial sensors MTx Xsens
Company (Xsens Ic, Netherlands) which comprised
of a gyroscope, an accelerometer and a
magnetometer, which allowed us to know the
position in Cartesian space. For this application we
used 5 inertial sensors located on the head, trunk,
arm, forearm and hand. The captured inertial sensor
data and UL anthropometric data was used to
develop a biomechanical model that has been
previously reported (Gil-Agudo et al., 2011).
The kinematic assessment protocol consists of
the performing of one test, The Evaluation Session,
described as follows:
- Evaluation Session: The principal objective is to
assess the patient's functional capacity. This is
carried out by recording the kinematic variables for
the different degrees of freedom during the
execution of analytical movements of the UL. The
ranges of motion (ROM) of the shoulder, elbow and
wrist joints were analysed with MATLAB
®
(MATLAB R2009a, 2009), a mathematics software
tool.
Neurological examinations of all the patients were
performed according to the ASIA standards (Marino,
et al., 2003). The right and left motor indexes were
determined from the sum of the muscle strength
(MB) of C5 and T1 segments from right and left
extremities, respectively. For each motor index,
scores ranged from 0 to 25.
The functional examination was carried out
using four scales. FIM consists of 18 items
organized into six categories, four corresponding to
motor functions (self-care items, sphincter control,
mobility items, and locomotion) and two
corresponding to cognitive functions
(communication, psychosocial, and cognitive). The
lowest and highest scores of the total ranged from 18
to 126 (Hamilton et al., 1991). The second scale was
SCIM II that has 16 items divided into three
functional areas: self-care, respiration and sphincter
management, and mobility. Total score can vary
from 0 (minimal) to 100 (maximal) (Catz et al.,
1997). The Barthel Index (BI) consists of 10 tasks:
eating, bathing, grooming, dressing, bowels, bladder,
toilet use, transfers (bed to chair and back), mobility
(on level surfaces) and stairs. Total score is from 0
to 100 (Mahoney and Barthel, 1965.). The fourth
assessment scale was the UL part of Motor Index
(MI) that assesses the power and range of active
movement, which are rated for shoulder abduction,
elbow flexion, and pinch between the thumb and
index finger. Each movement is rated on a 0-100
point scale
(Demeurisse et al., 1980).
2.5 Data Analysis
The Pearson correlation coefficient was used to
correlate kinematic variables (shoulder, elbow and
wrist ROM) with clinical and functional variables. A
significance level of p less than 0.05 was used. To
compare the mean values of the kinematics, clinical
and functional variables between groups, the
nonparametric Mann-Whitney test was used. The
statistical analysis was done with the program SPSS
17.0
17
.
Clinical,FunctionalandKinematicCorrelationsusingtheVirtualRealitySystemToyra®asUpperLimbRehabilitation
ToolinPeoplewithSpinalCordInjury
83
3 RESULTS
Since no differences were found in any of the
analyzed variables, obtained from the first
assessment session using the Toyra® system and the
battery of scales, we conclude that the initial
functional status was similar between the groups.
When comparing the kinematic data, obtained
from the Toyra®, of both groups after treatment we
found a statistically significant difference (p=0.039)
in the wrist extension ROM. No statistically
significant difference was obtained in any of the
clinical and functional variables. However, notable
differences, more than one point between the groups,
were found when the pre and post evaluations were
compared using the parameters for BI and MI
dominant arm, showing higher scores for the IG.
Furthermore, for most of the items in the follow-up
evaluation (3 of the 5 items) and the ´follow-up
after´, obtained from the subtraction of the ´after´
from the ´follow-up´, (4 of the 4 items) patients from
the IG presented larger scores than those from the
CG (Tables 2 and 3).
Positive correlations between clinical and
functional measures and the kinematic variables
were found in the CG before treatment: FIM and
elbow flexion complete (r=0.966, p=0.034), MB and
elbow flexion complete (r=0.971, p=0.029), MI and
elbow flexion complete (r=0.999, p=0.001); after
treatment: MI and elbow extension (r=0.995,
p=0.005);and in the follow up evaluation: SCIM and
elbow extension (r= 0.998, p=0.041), MB and wrist
supination (r=0.999, p=0.024).
In relation to the IG we also found positive
correlations between clinical and functional
measures and the kinematic variables before
treatment: MB and wrist extension (r=0.642,
p=0.045), MB and wrist ulnar deviation
(r=0.654,p=0.040), MI and shoulder abduction by
steps (r=0.610, p=0.046), BI and shoulder flexion by
steps (r=0.618, p=0.043), BI and wrist extension
(r=0.611, p= 0.046); after treatment: MB and wrist
pronation (r=0.649, p=0.031), FIM and wrist
pronation (r=0.747, p=0.013); and in the follow up
evaluation: SCIM and elbow flexion by steps
(r=0.808, p=0.028).
Negative correlation in the IG between FIM and
wrist extension after treatment (r=-0.665, p=0.036)
were obtained in the IG. The results are shown in
Table 4.
Table 2: Clinical and functional parameters in both groups before and after treatment program. The table shows the results
of each group (mean and standard deviation) and the differences between groups (p) in different stages of the protocol. The
parameter “Follow up – After” is obtained by subtracting "after treatment" from "follow-up". *Statistically significant
differences.
Before treatment After treatment
CG IG p CG IG P
SCIM [0-100]
25±9.6 24.42±7.24
0.851
29.83±6.17 27.75±4.91
0.605
FIM [18-126]
63±4.76 60.20±5.86
0.395
65.00±6.68 61.80±4.36
0.395
BI [0-100]
19.17±12.81 17.92±13.39
0.813
23.33±16.02 23.75±12.27
0.668
MB DOMINANT ARM
[0-25]
12±6.35 14.09±5.99
0.511
13.83±6.91 14.82±5.67
0.646
MI DOMINANT ARM
[0-100]
71±15.01 66.33±13.95
0.639
78.33±20.08 75.50±15.16
0.572
Follow-up Follow up – After
CG IG p CG IG P
SCIM [0-100]
26.00±4.58 36.29±8.75
0.052
-2.00±2.64 7.57±9.91
0.086
FIM [18-126]
59.67±3.51 65.57±6.87
0.203
-2.33±4.04 4.43±3.99
0.067
BI [0-100]
29.50±2.88 27.86±8.59
0.246
-3.33±2.88 -0.71±6.72
0.410
MB DOMINANT ARM
[0-25]
13.33±7.76 13.43±5.19
1.00
0.33±2.30 0.14±1.67
1.00
MI DOMINANT ARM
[0-100]
79.67±24.13 79.29±14.24
0.817
2.67±4.61 2.71±12.61
1.00
NEUROTECHNIX2013-InternationalCongressonNeurotechnology,ElectronicsandInformatics
84

Table 3: Kinematic variables in both groups before and after treatment program. The table shows the ROM results in each
group (mean and standard deviation) and the differences between groups (p) in different protocol stages.
Before treatment
CG IG p
abdshoulder_s
90.56±39.10 88.92±37.73
0.896
abdshoulder_c
93.67±34.49 101.46±45.63
0.896
fshoulder_s
139.96±63.84 133.70±47.91
0.794
fshoulder_c
130.32±60.73 129.21±41
1.000
felbow_s
129.69±15.04 116.14±23.99
0.361
felbow_c
130.31±14.58 121.77±13.46
0.361
exelbow
137.88±23.46 136.67±20.87
0.896
rotshoulder
121.24±40.02 103.05±29
0.361
exwrist
56.35±16.60 58.12±18.57
0.433
supwrist
138.89±20.20 148.73±69.94
0.361
pronwrist
38.11±21.33 54.33±11.66
0.192
rdwrist
28.33±12.42 25.19±8.86
0.602
udwrist
20.38±14.68 31.53±11.85
0.240
After treatment
CG IG p
abdshoulder_s
99.99±40.20 99.94±38.70
0.0808
abdshoulder_c
96.57±33.10 108.86±38.47
0.544
fshoulder_s
128.46±67.56 151.73±42.96
0.396
fshoulder_c
124.94±66.02 150.64±40.99
0.544
felbow_s
141.21±13.69 127.39±28.96
0.332
felbow_c
135.21±13.97 125.39±19.21
0.275
exelbow
141.89±20.40 141.01±29.08
0.903
rotshoulder
106.17±49.24 134.81±81.54
0.467
exwrist
50.19±12.70 74.39±25.39
0.039*
supwrist
149.66±29.98 143.57±30.33
0.716
pronwrist
34.04±11.91 47.67±22.63
0.332
rdwrist
40.92±29.58 38.73±17.24
0.903
udwrist
34.53±35.28 36.30±14.78
0.396
Follow-up
CG IG p
abdshoulder_s
80.35±21.21 126.57±46.54
0.305
abdshoulder_c
79.65±21.22 121.17±41.47
0.210
fshoulder_s
114.14±67.49 161.75±25.90
0.425
fshoulder_c
109.15±64.92 151.67±21.25
0.305
felbow_s
150.19±17.46 142.65±5.70
0.425
felbow_c
141.64±17.21 134.26±18.06
0.732
exelbow
154.43±37.08 145.53±22.23
0.732
rotshoulder
165.37±114.77 143±60.93
0.909
exwrist
56.89±4.74 66.56±14.47
0.138
supwrist
133.27±23.92 177.54±80.85
0.210
pronwrist
38.09±26.18 68.90±22.13
0.138
rdwrist
27.46±10.82 57.29±49.76
0.425
udwrist
27.17±9.92 34.42±19.04
0.732
abdshoulder_s: shoulder abduction by steps; abdshulder_c: shoulder abduction complete; fshoulder_s:
shoulder flexion by steps; fshoulder_c: shoulder flexion complete; felbow_s: elbow flexion by steps;
felbow_c: elbow flexion complete; exelbow: elbow extension; rotshoulder: shoulder rotation; exwrist:
wrist extension; supwrist: wrist supination; pronwrist: wrist pronation; rdwrist: wrist radial deviation;
udwrist: wrist ulnar deviation.
Clinical,FunctionalandKinematicCorrelationsusingtheVirtualRealitySystemToyra®asUpperLimbRehabilitation
ToolinPeoplewithSpinalCordInjury
85

Table 4: Statistically significant differences found in the
correlation between clinical and functional variables with
kinematic variables in CG (a) and IG (b) in the different
protocol stages.
SCIM FIM MB MI
felbow_c
r:0.971
p:0.029 b
r:0.971
p:0.029 b
r:0.999
p:0.001 b
exelbow
r:0.998
p:0.041f
r:0.995
p:0.005 a
supwrist
r:0.999
p:0.024 f
a. CG correlations (r= Pearson correlation coefficient; p=
significance level). Protocol stages: b= before treatment, a=after
treatment, f=follow up.
SCIM FIM BI MB MI
abdshoulder_s
r:0.610
p:0.046b
fshoulder_s
r:0.618
p:0.043b
felbow_s
r:0.808
p:0.028
f
exwrist
r:0.611
p:0.046b
r:0.642
p:0.045b
prowrist
r:0.747
p:0.013a
r:0.649
p:0.031a
udwrist
r:0.654
p:0.040b
b. IG correlations (r= Pearson correlation coefficient; p=
significance level). Protocol stages: b= before treatment, a=after
treatment, f=follow up.
4 CONCLUSIONS AND FUTURE
WORK
The present study shows a work based on the
validity of the VR system Toyra®, in measuring the
changes in kinematic variables by comparing them
with clinical and functional results. We have also
measured the efficacy of this system as a
rehabilitation tool. The VR system Toyra® has
proved to be valid and consistent not only as an
assessing tool, but also as a rehabilitation device.
In a previous study
(Gil-Agudo et al., 2012), we
found trends indicating improvements in kinematic,
functional and clinical variables after treatment in
the IG. Statistically significant differences were
found between the groups from the results of a test
that assessed the manipulative skill, coordination
and fine grip. The trend obtained from the patients in
this study, where the values of the functional and
clinical upper limb parameters were increased in the
IG, corroborating the findings from the preliminary
study. Muscle strength could be a good indicator of
functional and clinical conditions of patients with
tetraplegia. Some researchers (Beninato et al., 2004),
have shown the specific contribution that each
muscle group has on the accomplishment of motor
tasks, assessed by FIM, in patients with low cervical
lesions. The positive trends found in the scales that
assess both power and range of active movements
(MI) and activities of daily living (BI), after the
ADLs training with the VR system, support these
theories.
It is important to highlight that the IG maintains
better results, in the clinical and functional scales,
than CG from the results obtained by subtracting
after treatment from follow-up. This means that
people in IG continue improving even after the
treatment, while CG patients lost most of the
improvements.
In addition, there was a statistically significant
difference between groups after treatment for the
wrist extension ROM. The Toyra® system requires
from the patient through the execution of arm and
hand activities, like eating with a spoon or combing
their hair wrist movements. Our proposed hypothesis
is that due to this training, the patients have
increased their hand dexterity.
We also think that the small sample size and the
short time of intervention with the Toyra® system
are contributing factors to the lack of statistical
significance in the others scales.
In this study, the correlations between functional
and clinical variables and kinematic parameters, in
different treatment times, were studied in order to
know the kind of relation and the system
effectiveness as measure tool.
First of all, we want to highlight that we have
found correlations in every evaluation stage and in
all the kinematic, clinical and functional variables in
both groups.
The functional scales used in this study (FIM,
SCIM and BI) showed positive correlations with the
kinematic variables and corroborate the findings of
studies that present a relationship between functional
and kinematic variables
(Tsao and Mirbagheri,
2007).
The negative correlation found between FIM and
kinematic variables after treatment in the IG could
be due to the limitations of the FIM with regards to a
subpopulation of SCI where the motor score is not
capable of adequately discriminating the
neurological level. This could be explained by the
fact that it is not evaluation specific for SCI
(Cacho
et al., 2011).
The correlation between strength and kinematic
parameters, measured with MB, indicate that muscle
NEUROTECHNIX2013-InternationalCongressonNeurotechnology,ElectronicsandInformatics
86

function in SCI has an important role in
characterizing movements of those patients.
This leads us to believe that both strength and
kinematics performance, are requirements for a
smooth and harmonious movement
(Cacho et al.,
2011).
The relationship between MI and kinematic
variables showed the strongest positives
correlations. This may be due to the fact that as the
motor level is higher, the ROM is bigger.
In most cases, the correlations indexes (CCI) are
higher than 0.70, which is the lower limit to be
considered reliable. Furthermore, there are several
parameters with a CCI higher than 0.80 which
indicates a very reliable correlation
(Baydal-
Bertomeu et al., 2010).
This study allows us to open a new area of
research based on the validation of different motor
capture systems not only as kinematic but also
functional tools, making it possible to measure
activities of daily living in an objective way. These
results can inform the clinicians on the efficacy of
the different rehabilitation methods and their impact
on the patients’ functionality.
Another future field of work is the development
of functional motor models, for use with robotic and
virtual reality rehabilitation programs based on
activities of daily living as well as the opportunity to
adapt each treatment to suit the individual functional
characteristics of the patients.
ACKNOWLEDGEMENTS
We thank the consortium including Foundation
Rafael del Pino, Foundation of the Spanish National
Hospital for Paraplegic Research and Integration
(FUHNPAIIN) and INDRA Systems for funding this
research.
REFERENCES
Alt Murphy, M., Sunnerhagen, K. S., Johnels, B., Willén,
C. J. (2006). Three-dimensional kinematic motion
analysis of a daily activity drinking from a glass: a
pilot study. Journal of neuroengineering and
rehabilitation, 3, 18.
Baydal-Bertomeu, J. M., Viosca-Herrero, E., Ortuño-
Cortés, M. A., Quinza-Valero, V., Garrido-Jaen, D.,
Vivas Broseta, M. J. (2010). Estudio de la eficacia y
fiabilidad de un sistema de posturografıa en
comparación con la escala de Berg. Rehabilitación,
44(4), 304–310.
Beninato, M., O’Kane, K. S., Sullivan, P. E. (2004).
Relationship between motor FIM and muscle strength
in lower cervical-level spinal cord injuries. Spinal
Cord, 42, 533–540.
Cacho, E. W., de Oliveira, R., Ortolan, R. L., Varoto, R.,
Cliquet, A. Jr. (2011). Upper limb assessment in
tetraplegia: clinical, functional and kinematic
correlations. International journal of rehabilitation
research, 34(1), 65-72.
Catz, A., Itzkovich, M., Agranov, E., Ring, H., Tamir, A.
(1997). SCIM-spinal cord independence measure: a
new disability scale for patients with spinal cord
lesions. Spinal Cord, 35, 850 -856.
Demeurisse, G., Demol, 0., Rolaye, E. (1980). Motor
evaluation in vascular hemiplegia. European
neurological journal, 19, 382-9.
Eng, K., Siekierka, E., Pyk, P., et al. (2007). Interactive
visuo-motor therapy system for stroke rehabilitation.
Medical & biological engineering & computing,
45(9), 901-7.
Gil-Agudo, A., Del Ama-Espinosa, A., De los Reyes-
Guzmán, A., Bernal-Sahún, A., Rocón, E. (2011).
Applications of upper limb biomechanical models in
spinal cord injury patients (internet monograph) in: V.
Klika, Ed. Rijeka (Croatia): Biomechanics in
Applications (pp. 125-164).
Gil-Agudo, A., Dimbwadyo, I., Peñasco-Martín, B., De
los Reyes-Guzmán, A., Bernal-Sahún, A., Berbel-
García, A. (2012). Experiencia clínica de la aplicación
del sistema de Realidad Virtual TOYRA en la neuro-
rehabilitación de pacientes con lesión medular.
Rehabilitación, 46(1), 41-48.
Hamilton, B. B., Laughlin, J. A., Granger, C. V., Kayton,
RM. (1991). Interrater Agreement of the Seven Level
Functional Independence Measure (FIM). Archives of
physical medicine and rehabilitation, 72, 790.
Harvey, L. A., Batty, J., Jones, R., Crosbie, J. (2001).
Hand function of C6 and C7 tetraplegics 1 - 16 years
following injury. Spinal Cord, 39(1), 37-43.
Mahoney, F. I., Barthel, D. W. (1965). Functional
evaluation: the Barthel Index. Maryland state medical
journal, 14, 61-65.
Marino, R. J., Barros, T., Biering-Sorensen, F., Burns, S.
P., Donovan, W. H., Graves, D. E., et al. (2003).
International standards for neurological classification
of spinal cord injury. The journal of spinal cord
medicine (Vol. 26, pp. S50-56).
Matrix House, Cambrige, UK.
Oess, N. P., Wanek, J., Curt, A. (2012). Design and
evaluation of a low-cost instrumented glove for hand
function assessment. Journal of neuroengineering and
rehabilitation, 9, 2.
Snoek, G. J., IJzerman, M. J., Hermens, H. J., Maxwell,
D., Biering-Sorensen, F. (2004). Survey of the needs
of patients with spinal cord injury: impact and priority
for improvement in hand function in tetraplegics.
Spinal Cord, 42 (9), 526-32.
SPSS Statistics 17.0. SPSS Inc, Chicago, IL, USA. (2008).
Stewart, J. C., Yeh, S. C., Jung, Y., Yoon, H., Whitford,
M., Chen, S. Y., et al. (2007). Intervention to enhance
skilled arm and hand movements after stroke: A
Clinical,FunctionalandKinematicCorrelationsusingtheVirtualRealitySystemToyra®asUpperLimbRehabilitation
ToolinPeoplewithSpinalCordInjury
87

feasibility study using a new virtual reality system.
Journal of neuroengineering and rehabilitation, 23(4),
21.
Tsao, C. C., Mirbagheri, M. M. (2007). Upper limb
impairment associated with spasticity in neurological
disorders. Journal of neuroengineering and
rehabilitation, 4, 45.
Van Tuijl, J. H., Janssen-Potten, Y. J., Seelen, H. A.
(2002). Evaluation of upper extremity motor function
tests in tetraplegics. Spinal Cord, 40(2), 51-64.
Weiss, P. L., Kizony, R., Feintuch, U., Katz, N. (2006).
Virtual reality in neurorehabilitation. En: Selzer M,
Clarke S, Cohen L, Duncan P, Gage F, eds. Textbook
of neural repair and rehabilitation (pp. 182-97).
Cambridge: University Press,.
Wyndaele, M., Wyndaele, J. J. (2006). Incidence,
prevalence and epidemiology of spinal cord injury:
what learns a worldwide literature survey? Spinal
Cord, 44(9), 523-9.
NEUROTECHNIX2013-InternationalCongressonNeurotechnology,ElectronicsandInformatics
88
