
Simultaneous Optical Stimulation and Electrophysiological Recordings
in Closed-loop Operation
Thoa Nguyen
1,3
, Ling Wang
1,3
, Henrique Cabral
1,4
, Georges Gielen
2,3
, Francesco Battaglia
1,4
and Carmen Bartic
1,3
1
NERF, Leuven, Belgium
2
Imec, Leuven, Belgium
3
Katholieke Universiteit Leuven, Leuven, Belgium
4
Radboud Universiteit Nijmengen, Nijmengen, Netherlands
1 INTRODUCTION
Closed-loop brain computer interfaces are rapidly
progressing due to their application in funda-
mental neuroscience and prosthetics implemented
(Hatsopoulos and Donoghue, 2009; Lebedev and
Nicolelis, 2006). The integration of optical stim-
ulation and electrophysiological recordings, on one
hand, brings the advantage of cell-type selectivity. On
the other hand, it provides an alternative solution to
the stimulation-induced artifacts, a challenge in elec-
trical stimulation (Zhang et al., 2009; Zhang and Oert-
ner, 2007; Wininger et al., 2009).
In this contribution, we describe a prototype al-
lowing simultaneous optical stimulation and electro-
physiological recordings in a closed-loop manner.
The prototype is implemented with online spike de-
tection and classification for selective cell-type stim-
ulation.
2 METHODS
2.1 System Architecture
The implemented system is based on commercial off-
the-shelf electronics with three functional parts: (1)
data acquisition, (2) LED stimulation, and (3) control
software (see Fig. 1).
The acquisition circuitry measures the brain activ-
ity collected on 32 channels with respect to the skull
reference electrode. The on-board amplifier (Intan
chip - RHA2132) amplifies and then multiplexes the
signal before delivering it to the analog-to-digital con-
verter (AD7980). The filters integrated in the ampli-
fier are set by external resistors to record the broad-
band signal, i.e. 0.2 - 5000 Hz. The acquisition head-
stage is digitally interfaced with the digital I/O board
(Data acquisition card (DAQ) - PCI 6259M).
The fiber-coupled LED light source (Thorlab) is
controlled by TTL voltage pulses. The pulses are de-
livered from the analog output of the DAQ card with
pre-defined amplitude and duration.
Our custom developed software controls the ac-
quisition, triggers the stimulation, and analyzes the
recorded signals. The software is implemented on
LabVIEW platform and integrates signal processing
code written in Matlab. Data from the headstage are
transferred to the computer’s memory through a high-
speed acquisition loop. In parallel to that, a consumer
loop stores and analyzes the data.
A data processing sequence for spike detection
and classification is defined for the real-time execu-
tion. The implemented spike detection recognizes
possible spikes by an adaptive threshold-based algo-
rithm (Quiroga et al., 2004) applied to the band-pass
filtered data (300 - 5000 Hz). Next, the detected
signals are correlated with previously extracted tem-
plates, which were defined offline from a baseline
recording period at the beginning of the session. In
this first prototype, we employed a simplest form of
template matching, i.e. a dot-product, and assigned
the spikes to the cluster resulting in the maximum cor-
related value.
2.2 Microdrive with Optical Fibers
A microdrive is built based on a previous design
(Kloosterman et al., 2009) (see Fig. 1). It hosts two
separate tetrode bundles with 12 recording tetrodes
(Wilson and McNaughton, 1993) and one optic fiber
each, allowing recording and optically stimulating
neural activity from two different brain regions. Each
tetrode consists of a twisted bundle of four or eight
polyimide-insulated microwires, fused and cut to cre-
ate a blunt tip.
Nguyen T., Wang L., Cabral H., Gielen G., Battaglia F. and Bartic C..
Simultaneous Optical Stimulation and Electrophysiological Recordings in Closed-loop Operation.
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
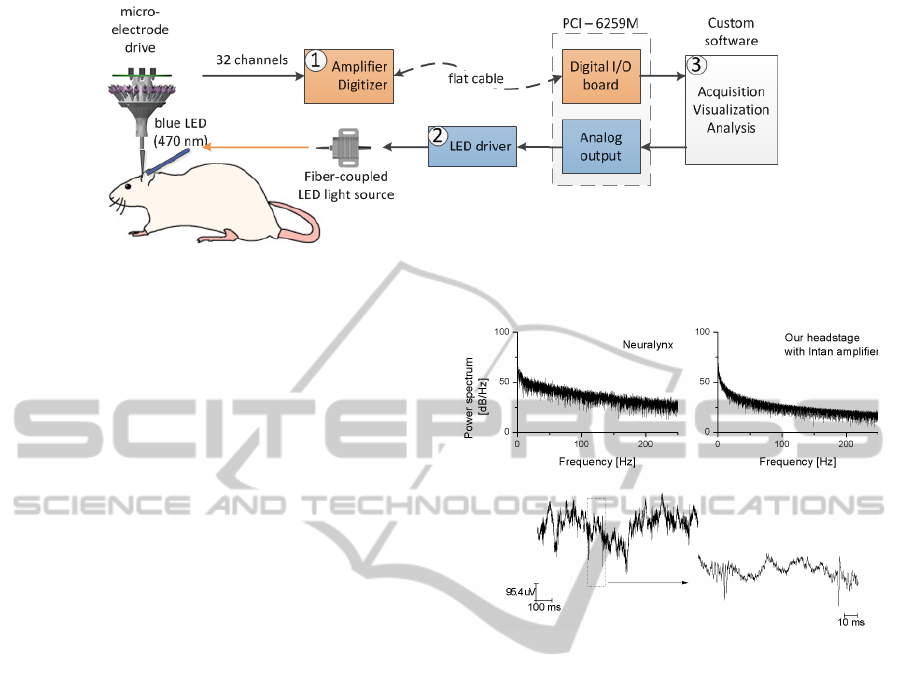
Figure 1: Schematic of experimental setup. The neural signals acquired from 32 channels (1) are detected and classified
online (3) for LED stimulation (2).
2.3 Animal Surgery
All in vivo measurements were performed in the dor-
sal hippocampus of awake rats (adult male Sprague-
Dawley rats weight >350 g). Experiments were car-
ried out in accordance with protocols approved by
the local University animal ethics committee and in
accordance with the European Communities Council
Directive of November 24, 1986 (86/609/EEC).
During the implantation, rats were anesthetized
with 1.5-3% inhaled isoflurane and given a subcu-
taneous injection of buprenorphine (0.05 mg/kg) to
minimize pain. The craniotomy was made over the
right dorsal hippocampus, centered at 3.5 mm poste-
rios and 2.8 lateral to bregma, and then sealed with
cyanoacrylate glue. The rats returned to their nor-
mal housing, and had 5-8 days of recovering before
the first recording session. In the mean time, tetrodes
were lowered while monitoring activity in order to at-
tain correct position.
3 RESULTS
The prototype was first evaluated in terms of the
recording capability in awake rats. Figure 2(bottom)
displays a representative recorded trace from a chan-
nel in the hippocampus. When comparing the base-
line measurements between our custom headstage and
the commerical Neuralynx system, we obtained a
similar power spectrum (Figure 2(top)), indicating a
comparable noise level between two systems.
The system’s functionality has been evaluated in
three sessions with the awake rat (Figure 3). The
brain activities were measured and transferred from
the headstage to the computer every 8 ms. The sig-
nals are then processed by the embedded Matlab code
to detect spikes. After calculating the threshold in
the current process, a 32-point waveform segment (8
pre- and 24-post) around the detected peak was ex-
Figure 2: Multi-taper spectrum of the recorded signal by the
commerical Neuralynx system (top, left), and by our proto-
type (top, right). A segment of recordings in a channel with
the prototype and its zoom-out are shown in the bottom.
tracted. The extracted waveforms for every tetrode (4
channels) were correlated with a set of pre-established
waveform templates. Spikes were assigned to the
template with the highest correlation score. In Figure
3, when the neuron 16 fires, a TTL pulse of 2 ms is
triggered and delivered to the optical fiber to interfere
with its activity.
4 DISCUSSION
We have successfully demonstrated the closed-loop
operation of our prototype. The optical stimulation is
selectively triggered based on the results of the online
spike sorting. The processing sequences, from ac-
quisition to spike detection, spike classification, and
stimulation, operates in real-time (frame rate of 8 ms)
for at least 8 tetrodes (32 channels) on a standard
workstation.
Although the implemented processing sequence is
the simplest form of template matching, this approach
provides a basic single-unit discrimination for non-
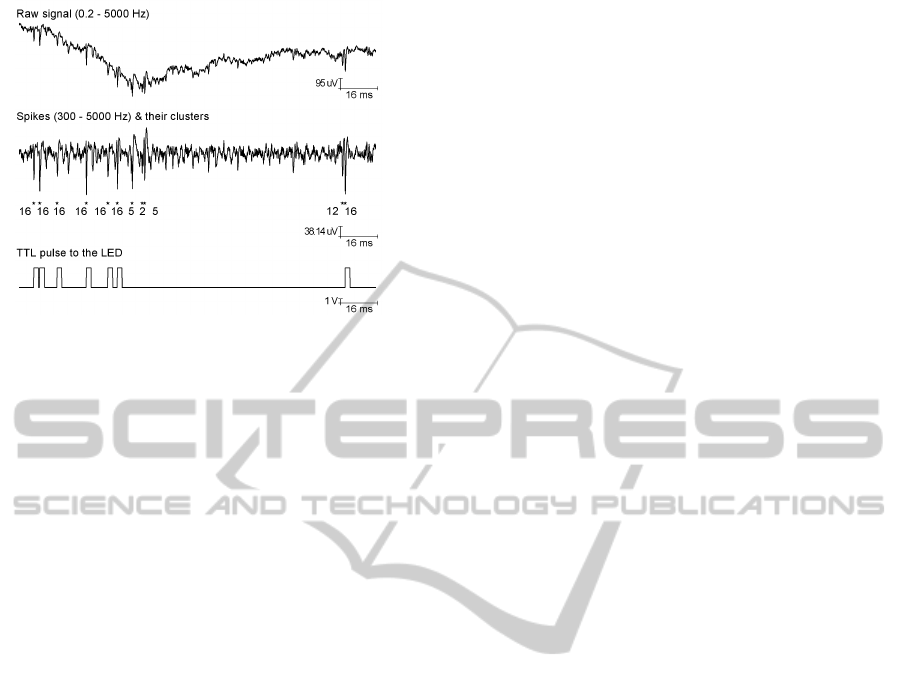
Figure 3: Closed-loop operation of our system. The ac-
quired raw data (0.2 - 5000 Hz) is filtered (300 - 5000 Hz),
amplitude-thresholded and classified to clusters with high-
est score after template matching. The LED is triggered
whenever cell 16 fires.
overlapping spike events. More accurate algorithms,
that involve matrix to vector multiplications, such as
Principal Component Analysis, are tested and should
be optimized to cope with the speed requirements.
ACKNOWLEDGEMENTS
This work has been supported in part by the EN-
LIGHTENMENT project that acknowledges the fi-
nancial support of the FET program within the FP7
for Research of the EC.
REFERENCES
Hatsopoulos, N. G. and Donoghue, J. P. (2009). The sci-
ence of neural interface systems. Annual Reviews of
Neuroscience, 32:246–266.
Kloosterman, F., Davidson, T. J., Gomperts, S. N., Lay-
ton, S. P., Hale, G., Nguyen, D. P., and Wilson, M. A.
(2009). Micro-drive array for chronic in vivo record-
ing: drive fabrication. Journal of visualized experi-
ments, page e1094.
Lebedev, M. A. and Nicolelis, M. A. (2006). Brain-machine
interfaces: past, present and future. Trends in Neuro-
sciences, 29:536–546.
Quiroga, R. Q., Nadasdy, Z., and Ben Shaul, Y. (2004). Un-
supervised spike detection and sorting with wavelets
and superparamagnetic clustering. Neural Comput.
Wilson, M. A. and McNaughton, B. L. (1993). Dynamics
of the hippocampal ensemble code for space. Science,
261:1055–1058.
Wininger, F. A., Schei, J. L., and Rector, D. M. (2009).
Complete optical neurophysiology: toward optical
stimulation and recording of neural tissue. Appl op-
tics, 48.
Zhang, J., Laiwalla, F., Kim, J. A., Urabe, H., Wagenen,
R. V., Song, Y.-K., Connors, B. W., Zhang, F., Deis-
seroth, K., and Nurmikko, A. V. (2009). Integrated
device for optical stimulation and spatiotemporal elec-
trical recording of neural activity in light-sensitized
brain tissue. Journal of Neural Engineering, 6.
Zhang, Y.-P. and Oertner, T. G. (2007). Optical induction
of synaptic plasticity using a light-sensitive channel.
Nature Methods, 4:139–141.
