
Transcranial Direct Current Stimulation Improves the Cycling
Performance but Does Not Alter Neuromuscular Function
tDCS, Cycling Performance and Neuromuscular Function
Leandro Ricardo Altimari
1
, Marcelo Vitor-Costa
1
, Henrique Bortolotti
1
and Nilo Massaru Okuno
2
1
Group of Study and Research in Neuromuscular System and Exercise, Physical Education and Sport Center,
Londrina University State, Londrina, Paraná, Brazil
2
Department of Physical Education, Ponta Grossa University State, Ponta Grossa, Paraná, Brazil
1 OBJECTIVES
The main neurophysiologic mechanisms that
determine performance in physical activities or
sports are not well understood. This is mainly due to
the lack of technology that permits the study of the
human brain in vivo. In the last decades some
neuromodulation techniques have been developed
and, among them, the transcranial direct current
stimulation (tDCS) has been attracting attention as it
is easily applicable and permits to carry out well
controlled studies in humans, and has been shown to
be a strategy to enhance physical and mental
performance in sports (Davis, 2013).
Therefore, the objective of the present study was
to investigate the effects of anodic tDCS on the
physical performance and neuromuscular function in
cycling exercise.
2 METHODS
A total of 11 physically active subjects aged 26 ± 4
years, weighing 77 ± 15 kg and 177 ± 3 cm tall
participated in study. Initially all subjects performed
an incremental test in a cyclesimulator (model
Velotron DYNAFIT PRO™, RacerMate Inc., USA)
to determine the peak power (257 ± 35 W). In the
two subsequent visits to the laboratory the subjects
were randomly submitted to one of the two
stimulation conditions (Anodic tDCS or Placebo
tDCS) to verify their possible effect on a time to
exhaustion task at 80% of peak power (205 ± 28 W).
This study was approved by the local Institutional
Research Ethics Committee.
Stimulation was carried before each test during
13 min, with a current intensity of 2.0 mA. The time
between tDCS and the test was 10 min. Sessions
were separated by a minimal interval of 48h. We
used the 10-20 International System for EEG
electrode placement. The active electrode (9x4 cm)
was placed on the scalp having its center in the
region Cz 4.5 cm on each side of the head (purpose
of stimulating the motor cortex of both
hemispheres), and the reference electrode was
positioned over the bulge.
Additionally, the electromyographic (EMG)
signal of the muscle vastus lateralis (VL) was
monitored during tests after stimulation and
expressed in mean values of RMS - root mean
square (µV) and MF - median frequency (Hz) with a
5-second window period. For EMG signal
normalization a test for torque-speed [T–V test] was
used (Rouffet and Hautier, 2008).
For the recording the of EMG signal, was used
an electromyography model TeleMyo 2400TG2™
(NORAXON Inc., USA) and bipolar active EMG
electrodes (modelo TeleMyo 2400™, NORAXON
Inc., USA), with interelectrode distance fixed at 2
cm, which were placed in the right leg muscle and
fixed with a adhesive tape. Initially, a trichotomy
followed by asepsis with alcohol and curettage of
the electrode site, to reduce skin impedance, was
performed.
The localization of anatomical point for the
electrode placement on the analyzed muscle was
done according to the standardization proposed by
the Surface ElectroMyoGraphy for the Non-Invasive
Assessment of Muscles ISEK: International Society
of Electrophysiology and Kinesiology (SENIAM).
The sampling frequency of the EMG signals was
2.000 Hz. The signal passing limit was ±5 mV, and
the common-mode rejection ratio was 95 dB.
To obtain the values expressed in RMS, the raw
EMG signals were submitted to a band-pass digital
filter of 20 and 500 Hz and then rectified and
smoothed. The MF was determined using Fourier
analysis —‘‘Short-Time Fast Fourier Transform’’ —
and the signals were processed in the mathematical
simulation environment MatLab 7.0™ (Mathworks
Ricardo Altimari L., Vitor-Costa M., Bortolotti H. and Massaru Okuno N..
Transcranial Direct Current Stimulation Improves the Cycling Performance but Does Not Alter Neuromuscular Function - tDCS, Cycling Performance
and Neuromuscular Function.
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
Inc., USA). For statistical analysis of the data was
used paired Student t-test and ANOVA two-way.
Significance level was set at 5%.
3 RESULTS
The results demonstrated that there was an increase
(p<0.05) in exercise time when individuals received
Anodic tDCS (491 ± 100s) in comparison to Placebo
tDCS (407 ± 69s). These results were confirmed by
the size effect (anodic x placebo = 0.77). When the
magnitude-based inference was applied, the anodic
stimulation condition was most probably positive to
individuals when compared to placebo conditions.
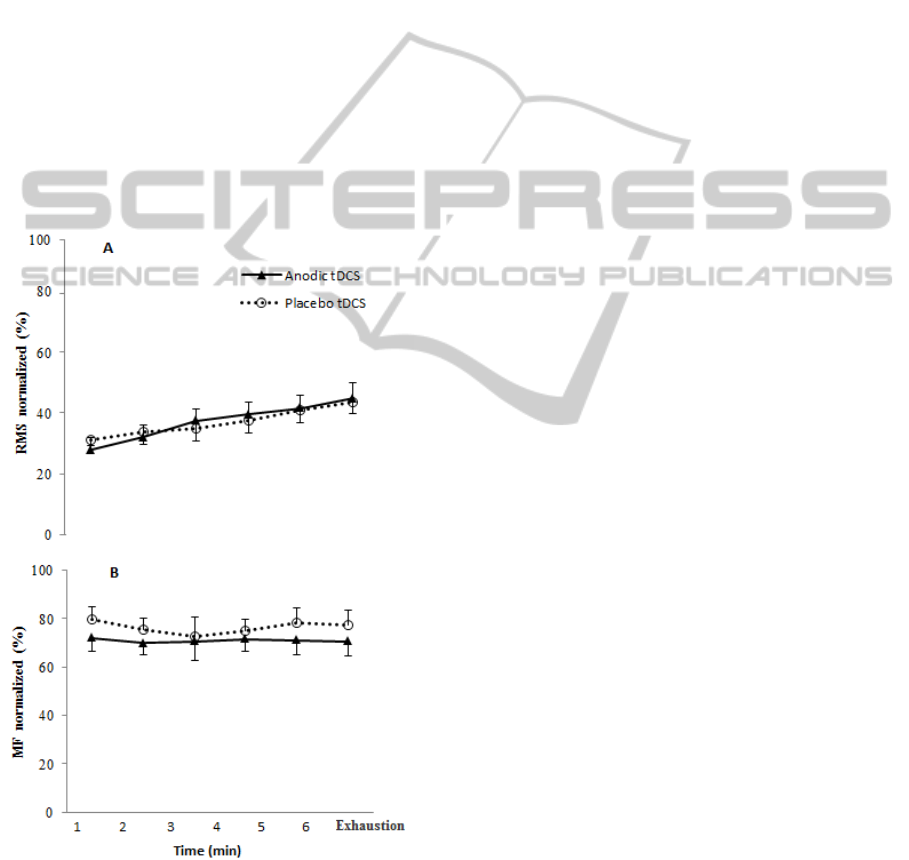
However, no significant differences were found
for the parameters of neuromuscular function - EMG
signals RMS (Figure 1A) and MF (Figure 1B) -
among the two experimental conditions.
Figure 1: EMG signal of the muscle vastus lateralis (VL)
monitored during the tests and expressed as mean values
of RMS and MF in the conditions Anodic tDCS or
Placebo tDCS.
4 DISCUSSION
The increase in exercise time with application of
anodic current has been shown previously in
isometric exercise of the upper limb. Cogiamanian et
al. (2007) showed that anodal stimulation (1,5 mA),
applied for 10 min after a fatigue test increased
tolerance to an exercise performed subsequent to
stimulation without changes in EMG. These findings
are in agreement with those found in this study.
Furthermore, some authors have shown that tDCS
can improve performance on other tasks increasing
muscle strength. Tanaka et al. (2009) have shown
that, in healthy subjects exerting pinch strength in
the toes, the anode tDCS causes increases in strength
both during and after 30 min of stimulation. More
recently, in another study with patients who have
suffered stroke, Tanaka et al. (2011) found an
improvement in knee extension strength of the
paretic leg during application of anodal tDCS, but
after 30 minutes there was no difference (Tanaka et
al., 2011). Thus, it may be concluded that anodic
TDCS increases exercise time. However, the
mechanisms responsible for the greater exercise
tolerance are speculative. It is possible that the
increase in intracortical facilitation causes the
individual to support longer in exercise.
REFERENCES
Cogiamanian, F., Marceglia, S., Ardolino, G., Barbieri, S.,
Priori, A. 2007. Improved isometric force endurance
after transcranial direct current stimulation over the
human motor cortical areas. European Journal of
Neuroscience. 26(1):242-249.
Davis, N. J. 2013. Neurodoping: Brain stimulation as a
performance-enhancing measure. Sports Med. [Epub
ahead of print] DOI: 10.1007/s40279-013-0027-z.
Rouffet., D. M., Hautier, C. A. 2008. EMG normalization
to study muscle activation in cycling. Journal of
Electromyography and Kinesiology. 18(5):866-878.
Tanaka, M., Takeda, K., Otaka, Y., Kita, K., Osu, R.,
Honda, M., Sadato, N., Hanakawa, T., Watanabe, K.
2011. Single session of transcranial direct current
stimulation transiently increases knee extensor force
in patients with hemiparetic stroke.Neurorehabilitation
and Neural Repair. 25(6):565-569.
Tanaka, S., Hanakawa, T., Honda, M., Watanabe, K. 2009.
Enhancement of pinch force in the lower leg by anodal
transcranial direct current stimulation. Journal of
Physiology Experimental Brain Research. 196(3):459-
465.
