
Computational Models of Populations of Motor Neurons
Jakob L. Dideriksen and Dario Farina
Department of Neurorehabiliation Engineering, Bernstein Focus Neurotechnology Göttingen,
Bernstein Center for Computational Neuroscience, Universitätsmedizin Göttingen, Göttingen, Germany
1 OBJECTIVES
In this paper, we will provide examples of how
computational models of motor neuron and muscle
activity can support basic and applied research on
human movement. Both examples focus on
pathological tremor. Tremor is a rhythmic,
involuntary oscillation of a limb and it is the most
prevalent movement disorder, symptomatic to e.g.
Parkinson’s Disease (Wenning et al., 2005). Tremor
implies a serious worsening of the quality of life,
also because the effect of the current treatments is
variable(Rascol et al., 2000).
First, we show how models can be applied in the
development of a rehabilitation device for
suppressing pathological tremor (Objective 1). The
proposed tremor rehabilitation system relied on
modulation of spinal neuron excitability of the
tremorogenic motor neurons using homonymous
excitation and reciprocal inhibition evoked by
electrical stimulation of peripheral type Ia nerves.
Successful implementation of this strategy implied
robust and precise on-line analysis of tremor
(Objective 1A) and stimulation parameter selection
(Objective 1B). Here, the Iterated Hilbert Transform
(IHT) applied to the surface EMG signals were
selected for tremor analysis (Dideriksen et al.,
2011).
Next, we demonstrate how models can enhance
the understanding of the underlying physiological
mechanisms of tremor (Objective 2), especially
aspects that cannotbe easily assessed experimentally.
Specifically, here we will address the contribution of
afferent feedback in tremor, which has been debated
in the literature (Rack and Ross 1986).
2 METHODS
The neuromechanical models applied to address the
two objectives consisted of a number of sub-models
and shared the same basic structure; however, the
level of model complexity required for addressing
each of the objectives determined how the various
sub-models were implemented.
2.1 Neuromechanical Models
The model was designed to reflect the characteristics
of an antagonist muscle pair acting on one limb in
one degree of freedom and consisted of a number of
sub-models interacting via one or more variables.
First, the activity of the motor neuron population
(spike trains) was determined based on the synaptic
input it received. Each motor neuron innervated a set
of muscle fibers (the motor unit). Each motor unit
was assigned a set of parameters describing its
contractile properties. Along with these properties,
the discharge rate determined the motor unit force.
The force of the muscles (the sum of the force
generated by all motor units) evoked the movement
of the limb. Proprioceptive activity was determined
by limb dynamics (muscle spindle; type Ia) and
muscle forces (Golgi tendon organs; type Ib) and
provided afferent feedback to the motor neurons.
Tremor was simulated by imposing a sine wave to
the motor neuron input.
For Objective 1A, a model of the motor unit
population and the force it generates was adopted
(Fuglevand et al., 1993), while the afferent feedback
was simulated as compound signals (Prochazka and
Gorassini, 1998). A model of the surface motor unit
action potentials(Farina et al., 2004) was used to
simulate the surfaceEMG signal (based on the motor
unit spike trains), that was used for tremor
estimation. For Objective 1B and 2 a more detailed
description of the single neuron behaviour (Cisi and
Kohn, 2008), a more advanced mechanical model
(Oguztoreli and Stein, 1982), as well as models
allowing the afferent feedback to be described as
spike trains (Mileusnic and Loeb, 2006) were
applied. In this way, simulation of axon action
potentials generated by surface stimulation was
made possible.
L. Dideriksen J. and Farina D..
Computational Models of Populations of Motor Neurons.
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
2.1.1 Model Validation
The validation for the model used for Objective 1A
relied on its ability to generate patterns of motor unit
spike trains, as observed in tremor. The key feature
for Objectives 1B and 2 were the capability of the
model to reflect the true spinal connectivity. For this
reason, experimentally used protocols for H-reflex
recruitment curves and estimation of reciprocal
inhibition strength were simulated.
2.1.2 Simulations
For Objective 1Athe surface EMG signal was
simulated in a variety of conditions, including
different contraction levels (0-20% MVC), tremor
frequencies (4-12 Hz), and tremor intensities.
Tremor identification was assessed by comparison
with the imposed neural oscillations. For Objective
1B stimulation amplitude, frequency, and timing
were varied. Tremor suppression was evaluated by
the integral power of the limb movement spectrum
with and without stimulation. For Objective 2,
simulations were carried out with and without
afferent feedback and the power spectra of the
neural drive in each situation were compared.
3 RESULTS
The motor unit inter-spike interval histograms in
simulated tremor were in agreement to those
experimentally observed previously (Christakos et
al., 2009) and the simulated H-reflex recruitment
curves and the strength of the reciprocal inhibition
were comparable to previous experimental results
(Wargon et al., 2006) (not shown).
3.1 Tremor Identification
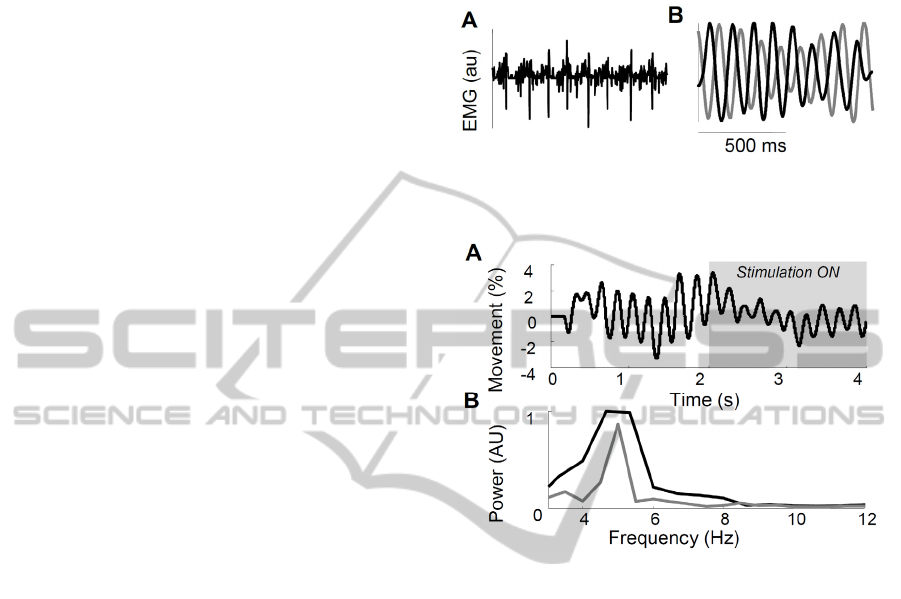
Figure 1 shows an example of the simulated surface
EMG signal during an 8-Hz tremor. Furthermore,
the oscillations imposed on the motor neuron
population (Figure 1B, black line) are shown,
superimposed with the estimation of the
tremorogenic input signal obtained from the surface
EMG using the IHT algorithm. The two signals were
similar, except the delay, caused by the delays of the
nervous system. Across all simulated conditions, the
estimation of the tremor amplitude was correlated
with the imposed amplitude (r
2
=0.52), and the
RMSE in estimation of frequency was 2.6 Hz,
mostly due to errors at high frequencies in
conditions with low tremor. This efficiency was
maintained in windows down to 500 ms, showing
the capability of the algorithm to drive tremor
suppression on-line (Dideriksen et al., 2011).
Figure 1: Simulated surface EMG (A) and imposed and
estimated tremor (black, grey respectively; B).
Figure 2: Simulated tremor with and without afferent
stimulation (grey box; A). Power spectrum (B) indicates
tremor suppression (grey line).
3.2 Tremor Suppression
Figure 2 depictsthe simulated tremor suppression
approach applied in a 2-s window during 5-Hz
tremor. The tremor amplitude was decreased by
63%, as also shown in the power spectrum
represented in the lower panel. The optimal
suppression was obtained at 60 Hz stimulation at an
intensity recruiting 22% of all Ia axons and no motor
neuron axons. Stimulation efficiency was highly
sensitive to the timing of the stimulation bursts with
respect to the imposed oscillations. Optimally a 15-
ms delay should be used, while deviations of more
than 25 ms involved tremor amplification, implying
the need for accurate tremor estimation algorithms.
3.3 Afferent Contribution to Tremor
Figure 3 shows the power spectra of the motor
neuron population output in simulations performed
with and without afferent feedback. At 6 Hz, the
afferent feedback enhanced the oscillations by up to
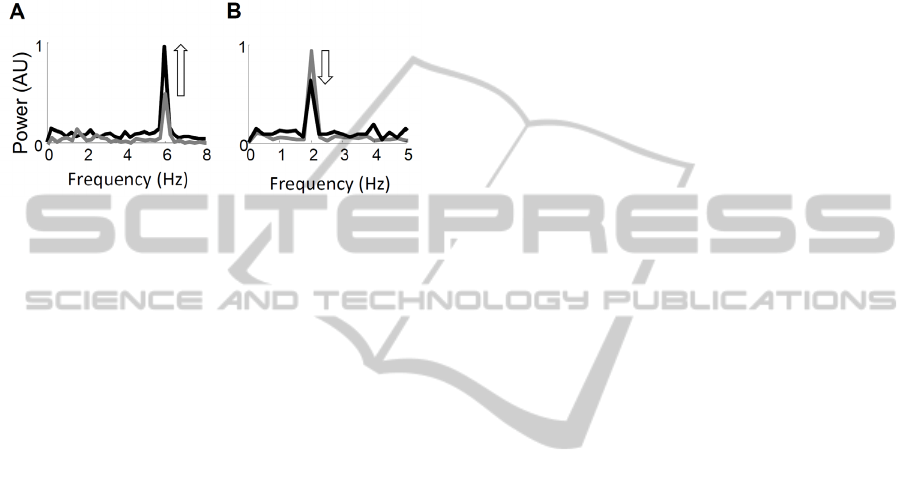
80%, while afferent feedback reduced tremor at 2 Hz
by 40%. This difference can be explained by the
neural delays. At low frequencies, the afferent
feedback arrives at the motor neuron almost exactly
out of phase with the imposed oscillations, and vice
versa at higher frequencies, due to faster
contractions. This observation may explain the
common occurrence of tremor in the 4-6 Hz range.
Figure 3: Power spectra of neural drive in simulations with
(grey) and without (black) afferent feedback with 6 Hz (A)
and 2 Hz (B) imposed.
4 DISCUSSION
Two examples of applications of neuromechanical
models were given, each highlighting different
advantages of using simulations to support
experimental tests. The first example demonstrated
how models can be applied to test the robustness of
rehabilitation techniques when experimental data is
sparse and when full parameter sensitivity analysis
in patients is not feasible. Last, the sensitivity of
internal physiological parameters on the motor
output was assessed, for neuromuscular properties
that cannot be measured experimentally.
ACKNOWLEDGEMENTS
This work was supported by the EU project
NEUROTREMOR (contract FP7-ICT-2011-7-
287739)
REFERENCES
Christakos, CN, Erimaki, S, al. Tremor-related motor unit
firing in Parkinson’s disease: implications for tremor
genesis. J Physiol 587.20, 2009.
Cisi, R. R. L., Kohn, A. F. Simulation system of spinal
cord motor nuclei and associated nerves and muscles,
in a web-based architecture. J. Comput Neurosci 55,
2008
Dideriksen, J. L., Gianfelici, F, et al., EMG-based
characterization of pathological tremor using the
iterated Hilbert transform. IEEE TBME 58(10), 2011
Farina, D, Mesin, L, et al., A surface EMG generation
model with multilayer cylindrical description of the
volume conductor. IEEE TMBE 51(3), 2004.
Fuglevand A. J., Winter, D. A., Patla, A. E. Models of
recruitment and rate coding organization in motor unit
pools. J Neurophysiol. 70(6), 1993.
Mileusnic, M. P., Brown, I. E., et al. Mathematical models
of proprioceptors I. Control and transduction in the
muscle spindle. J Neurophysiol 96, 2006.
Oguzteroli, M. N., Stein, RB. Analysis of a model for
antagonist muscles. Biol Cybern 45, 1982
Prochazka A, Gorassini, M. Models of ensemble firing of
muscle spindle afferents recorded during normal
locomotion in cats. J Physiol. 507.1, 1998.
Rack, P. M., Ross, H. F. The role of reflexes in the resting
tremor of Parkinson’s disease. Brain 109(1), 1986.
Rascol, O, Brooks, DJ, et al. A five year study of the
incidence of dyskinesia in patients with early
Parkinson’s disease who were treated with ropinirole
or levodopa. N Engl J Med 342(20), 2000
Wargon I., Lamy, J. C., et al., The disynaptic group I
inhibition between wrist flexor and extensor muscles
revisited in humans. Exp Brain Res 168, 2006
Wenning, G. K., Kiechl, S., et al. Prevalence of movement
disorders in men and women aged 50-89 years
(Bruneck study cohort): A population based study.
Lancet Neurol 4(12), 2005.
