
Design of Radioprobes for Pet and Spect Imaging
António Paulo
Radiopharmaceutical Sciences Group, IST/CTN, Instituto Superior Técnico, Universidade Técnica de Lisboa,
Estrada Nacional 10, km 139,7,2695-066 Bobadela LRS, Portugal
Keywords: Radiopharmaceuticals, Nuclear Cardiology, PET, SPECT.
Abstract: Nuclear Cardiology is an important and non-invasive tool for the clinical evaluation of patients with known
or suspected coronary artery disease (CAD), one of the leading causes of death in western countries. The
advancement of this field depends on the continuous improvement and development of equipment and
signal processing technologies. However, its success is primarily determined by the design and development
of new radiopharmaceuticals suitable for Single Photon Emission Computed Tomography (SPECT) and
Positron Emission Tomography (PET) imaging. Nuclear cardiology started in the mid-1970s with the use of
201
Tl-thallous chloride, which has been the firstly approved radiopharmaceutical for perfusion cardiac
imaging. Later on,
99m
Tc-Sestamibi was introduced and approved for clinical use. Nowadays,
99m
Tc-
Sestamibi is the most used radioprobe for SPECT cardiac imaging. In the case of PET, nuclear cardiology
still relies mainly on the use of [
18
F]-2-fluoro-2-deoxy-glucose, which is the gold standard metabolic tracer
for cardiac imaging. Until now, a variety of other SPECT and PET radioprobes have been tested as
radiopharmaceuticals for cardiac imaging. This contribution reviews representative examples of the
chemical/radiochemical strategies that have been used to design perfusion and target-specific
radiopharmaceuticals for cardiac imaging.
1 INTRODUCTION
Nuclear Medicine uses radioactive compounds for in
vivo imaging and therapeutic purposes. Such
compounds, named radiopharmaceuticals, are used
in very low concentration (10
-8
- 10
-12
M), having no
pharmacological effect.
For in vivo imaging there are two nuclear
modalities: Single Photon Emission Computed
Tomography (SPECT) and Positron Emission
Tomography (PET), which use or β
+
emitting
radionuclides, respectively (Table 1) (Correia,
2011); (Morais, 2012a); (Morais 2012b). In the case
of SPECT, the radionuclides decay by electron
capture (EC) or isomeric transition (IT) with
emission of penetrating photons having energies in
the range 100-250 KeV. In PET, the
+
particles
emitted by the radionuclide react with the electrons
from the medium releasing two photons of 511 KeV,
as a result of annihilation reactions. In both cases,
the resulting photons (100-250 KeV or 511 KeV) are
efficiently detected outside the body leading to
clinically useful medical images.
Table 1: Examples of radionuclides for medical imaging.
Nuclide Physical half-life Mode of decay (%) Application
99m
Tc
6.0 h IT (100) SPECT
123
I
13.2 h EC (100) SPECT
18
F
1.83 h
+
(97)
EC (3)
PET
11
C
20.3 min
+
(100)
PET
86
Y
14.7 h
+
(33)
EC (66)
PET
111
In
2.80 d EC (100) SPECT
67
Ga
3.26 d EC (100) SPECT
68
Ga
1.13 h
+
(99)
EC (10)
PET
62
Cu
9.67 min
+
(98)
EC (2)
PET
64
Cu
12.7 h
-
(40),
+
(19)
EC (41)
PET
117m
Sn
13.6 d IT (100) PET
Perfusion versus Target-specific Radiopharma-
ceuticals. The biodistribution of radiopharmaceu-
ticals can be determined by their chemical and
physical properties – perfusion radiopharma-
ceuticals - or by their biological interactions -
75
Paulo A..
Design of Radioprobes for Pet and Spect Imaging.
DOI: 10.5220/0004663400750078
In Proceedings of the International Congress on Cardiovascular Technologies (VisualCardio-2013), pages 75-78
ISBN: 978-989-8565-78-5
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
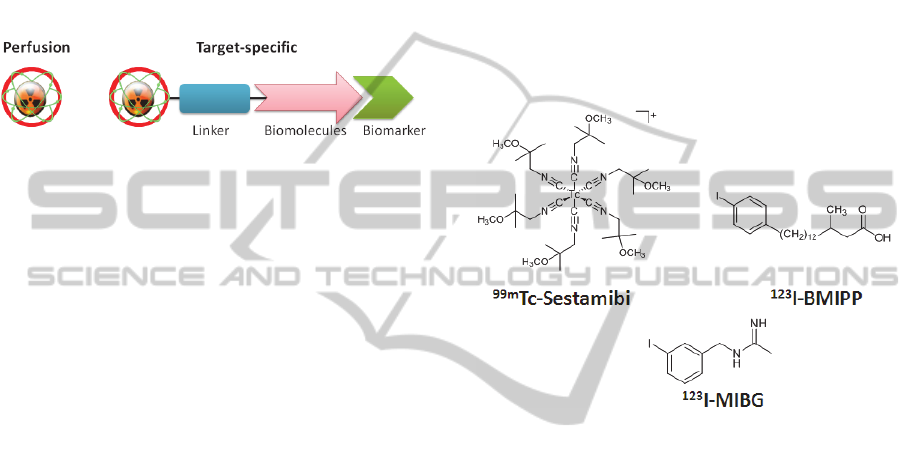
target-specific radiopharmaceuticals (Fig. 1). The
biological distribution of perfusion agents is
determined by blood flow and these agents target
high capacity systems, such as phagocytosis,
hepatocyte clearance, glomerular filtration. The
target-specific radiopharmaceuticals target low
capacity systems, and their biodistribution is
determined by specific protein interactions, for
example antigen, enzymatic or receptor-binding
interactions (Correia, 2011); (Morais, 2012a);
(Morais 2012b).
Figure 1: Schematic representation of perfusion and
target-specific radiopharmaceuticals.
In this contribution, we review representative
examples of the chemical/radiochemical strategies
that have been used to design perfusion and target-
specific radiopharmaceuticals for cardiac imaging.
This will comprise compounds labelled with
99m
Tc
or
18
F but also radioprobes containing less common
radionuclides like
123
I,
64
Cu or
117m
Sn.
2 RADIOPHARMACEUTICALS
FOR CARDIAC IMAGING
Nuclear Cardiology is an important and non-
invasive tool for the clinical evaluation of patients
with known or suspected coronary artery disease
(CAD), one of the leading causes of death in western
countries (Notghi, 2011); (Osborn, 2012). The
clinical importance of nuclear cardiology stems from
the unique advantages of Nuclear Imaging
Modalities (SPECT and PET), such as their high
intrinsic sensitivity, non–invasiveness and
specificity. The advances on nuclear cardiology
depend on the continuous improvement and
development of instrumentation and signal
processing technologies. However, its success is
primarily determined by the design, development
and validation of new, more sensitive and specific
radiopharmaceuticals.
2.1 SPECT Radioprobes
Nuclear cardiology started in the mid-1970s with the
use of
201
Tl-thallous chloride, which has been the
firstly approved radiopharmaceutical for perfusion
cardiac imaging by SPECT.
99m
Tc is the most widely
used SPECT radionuclide, due to its ideal nuclear
properties, low-cost and availability from
commercial
99
Mo/
99m
Tc generators. Later on, an
alternative
99m
Tc-based cardiac perfusion agent -
99m
Tc-Sestamibi (Fig. 2) – was introduced for
SPECT cardiac imaging, overcoming the limitations
associated with the unfavourable decay properties of
201
Tl (Maria, 2009).
99m
Tc-Sestamibi corresponds to
an organometallic Tc(I) compound, which is
synthesized in aqueous solution starting from the
Tc(VII) permetallate anion (
99m
TcO
4
-
) that is
reduced prior to its complexation by the isonitrile
ligands (Wackers, 1989).
Figure 2: Selected examples of SPECT radioprobes for
cardiac imaging.
Other radiometals, like
67
Ga,
111
In or the less
common
117m
Sn (see Table 1), are also relevant for
SPECT imaging. For instance, it has been recently
reported that the target-specific agent
117m
Sn-DOTA-
Annexin (TA) has potential for in vivo imaging of
vulnerable plaque (Strauss, 2013). This agent
corresponds to a Sn(II) complex with a macrocyclic
DOTA ligand functionalized with Annexin-V for the
targeting of phosphatidylserine (PS) that is
externalized in cells undergoing apoptosis.
Interestingly,
117m
Sn is a very promising
radionuclide for the development of theranostic
radiopharmaceuticals, as it decays via isomeric
transition with the emission of monoenergetic
conversion electrons. The pre-clinical evaluation of
117m
Sn-DOTA-Annexin in ApoE-/- mouse has
shown that this radioconjugate has some therapeutic
potential for the stabilization of vulnerable plaques
(Strauss, 2013).
Besides metal-based compounds, several
examples of radioiodinated molecules, such as
123
I-
BMIPP and
123
I-MIBG (Fig. 2), have also shown
promising for SPECT cardiac imaging. The methyl-
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
76

p-[
123
I]-iodophenyl-pentadecanoic acid (BMIPP) is a
radiolabeled branched fatty acid with the ability to
assess in vivo the viability of cardiac tissue, playing
an important role for identifying ischemia (Kontos,
2010). The meta-[
123
I]-iodobenzylguanidine (MIBG)
is a norepinephrine analogue that allow the in vivo
imaging of cardiac innervation, being useful to
assess the severity of heart failure and prognosis
(Tamaki, 2011).
123
I-BMIPP and
123
I-MIBG can be
obtained by isotopic exchange of stable
127
I by
123
I in
BMIPP and MIBG, respectively, using Cu(II) salts
as catalyst in the presence of a reducing agent. High
specific activity
123
I-MIBG is achievable by
electrophilic radioiodination of adequate stannylated
precursors (Vallabhajosula, 2011).
18
F‐FDG
18
F‐Flurpiridaz
62/64
Cu‐PTSM
Figure 3: Selected examples of PET radioprobes for
cardiac imaging.
2.2 PET Radioprobes
Until recently, the cyclotron-produced carbon-11
(
11
C) and fluorine-18 (
18
F) (Table 1) were the most
explored PET radionuclides on the design of
molecular imaging agents. Usually, labelling with
11
C frequently involves introduction of a [
11
C]methyl
group in the biomolecule via selective N- and O-
methylation (Ametamey, 2008). However, the very
short-life of this radionuclide (T
1/2
= 20.4 min) limits
its use to on-site cyclotron facilities, and requires
rapid one-step radiosynthesis. The longer
half-life of
18
F (T
1/2
= 110 min) allows for multistep
radiosynthesis, longer in vivo investigation and
commercial distribution to other clinical PET
centers. Radiofluorination reactions can be achieved
with either electrophilic or nucleophilic radioactive
fluoride. However, reactions with the less reactive
nucleophilic radiofluoride are more selective and
provide
18
F-labeled compounds in higher yields and
higher specific activity (Ametamey, 2008). For all
these reasons,
18
F remains the most used PET
radionuclide in radiopharmaceutical research.
In the particular case of nuclear cardiology PET
imaging relies mainly on the use of [
18
F]-2-fluoro-2-
deoxy-glucose, which has emerged several years ago
as the gold standard metabolic tracer for cardiac
imaging. (Strauss et al., 2013) Nowadays,
18
F-FDG
is produced worldwide in a large number of PET
facilities and under GMP conditions, based on a
nucleophilic reaction between a mannose triflate
precursor and [
18
F]fluoride.
More recently,
18
F-flurpiridaz (Fig. 2) a
structural analog of pyridaben obtained by
radiofluorination of a toluenesulfonate ester
precursor, started to be clinically evaluated as a PET
tracer for myocardial perfusion imaging.
18
F-
flurpiridaz targets the mitochondrial complex I (MC-
1), a mitochondrial protein found primarily in
myocardial cells, presenting a rapid uptake and slow
washout in cardiomyocytes. These characteristics
allow for a fast and sustained accumulation in the
heart (Ya, 2011).
There are several positron emitter radiometals
that are relevant the design of PET probes for
cardiac imaging, as is the case of
68
Ga and
62/64
Cu
(Table 1) (Cutler, 2013).
68
Ga is a positron emitter
readily accessible from the
68
Ge/
68
Ga generator,
offering the possibility to obtain on site a PET
radionuclide without needing the presence of a
nearby cyclotron. This possibility might open the
way to an important role for
68
Ga in PET imaging,
similarly to the role played by
99m
Tc in SPECT
imaging during the past few decades. Recently,
several cationic and lipophilic Ga(III) complexes
with hexadentate ligands have been synthesized and
pre-clinically evaluated as radioactive probes for
myocardial perfusion imaging (Hsiao, 2009).
Despite some encouraging results, none of the
reported Ga(III) complexes has shown potential to
be clinically evaluated as a PET probe for cardiac
imaging. So far,
62/64
Cu –PTSM has been the unique
metal-based compound that showed promising
biological properties as a PET perfusion imaging
agent. Although the mechanism is not fully
understood, it is considered that this small-sized,
neutral and lipophilic Cu(II) complex is retained in
the cells due to its intracellular reduction to Cu(I),
followed by release of the bis(thiosemicarbazone)
ligand (H
2
PTSM) (Paterson, 2011).
In summary, the chemical and structural
diversity of PET and SPECT radioprobes allowed
the design of several perfusion, metabolic and
target-specific agents for cardiac imaging, some of
them already in clinical use and others undergoing
clinical evaluation. Despite this success, there is still
room to investigate new radioprobes for nuclear
DesignofRadioprobesforPetandSpectImaging
77

cardiology, aiming at their translation from the
bench to the patient bedside.
REFERENCES
Ametamey, S. M., Honer, M., Schubiger, P. A.: Molecular
imaging with PET, Chem. Rev. 108 (2008) 1501-
1516.
Correia, J. D. G., Paulo, A., Raposinho, P. D., Santos, I.:
Radiometallated peptides for molecular imaging and
targeted therapy Dalton Trans 40 (2011) 6144-6167.
Cutler, C. S., Hennkens ,H. M., Sisay, N., Huclier-Markai,
S., Jurisson, S. S.: Radiometals for Combined Imaging
and Therapy, Chem. Rev. 113 (2013) 858-883.
Hsiao,Y-M., Mathias, C. J., Wey, S-P., Fanwick, P. E.,
Green, M. A.: Synthesis and biodistribution of
lipophilic and monocationic gallium radiopharma-
ceuticals derived from N,N′-bis(3-aminopropyl)-N,N′-
dimethylethylenediamine: potential agents for PET
myocardial imaging with
68
Ga, Nucl. Med. Biol., 36
(2009) 39-45.
Kontos, M. C., Dilsizian,V., Weiland, F., DePuey, G.,
Mahmarian, J. J., Iskandrian, A. E., Bateman, T. M.,
Heller, G. V., Ananthasubramaniam, K., Li, Y.,
Goldman, J. L., Armor, T., Kacena, K. A., LaFrance,
N. D., Garcia, E. V., Babich, J. W., Udelson, J. E.:
Iodofiltic Acid I 123 (BMIPP) Fatty Acid Imaging
Improves Initial Diagnosis in Emergency
DepartmentPatients With Suspected Acute Coronary
Syndromes, J. Am. Coll. Card. 56 (2010) 290-299.
Maria, L., Fernandes ,C., Garcia, R,. Gano, L., Paulo, A.,
Santos, I. C., Santos, I.: Tris(pyrazolyl)methane
99m
Tc
tricarbonyl complexes for myocardial imaging, Dalton
Trans (2009) 603–606.
Morais, G. R., Paulo, A., Santos, I.: Organometallic
Complexes for SPECT Imaging and/or Radionuclide
Therapy, Organometallics 31 (2012a) 5693-5714.
Morais, G. R., Paulo, A., Santos, I.: Synthetic Overview of
Radiolabeled Compounds for β-Amyloid Targeting,
Eur. J. Org. Chem. (2012b) 1279-1293.
Notghi, A., Low, C. S.: Myocardial perfusion
scintigraphy: past, present and future, Brit. J. Radiol.
84 (2011) S229–S236.
Osborn, E. A., Jaffer, F. A.: The Year in Molecular
Imaging, J. Am. Coll. Card. 58 (2012) 317-328.
Paterson, B. M., Donnelly, P. S.: Copper complexes of
bis(thiosemicarbazones): From chemotherapeutics to
diagnostic and therapeutic radiopharmaceuticals.
Chem. Soc. Rev. 40 (2011) 3005–3018.
Strauss, H. W., Narula, J., Orellana, P., Jaimovich, R.,
Stevenson, N., Gonzales, G., Srivastava, S.: Targeting
of vulnerable plaque using [tin-117m]-DOTA-
annexin, J Nucl Med. 54 (Supplement 2) (2013) 1667.
Tamaki, N.; Yoshinaga, K.: Novel iodinated tracers,
MIBG and BMIPP, for nuclear cardiology, J. Nucl.
Cardiol. 18 (2011) 135-143.
Vallabhajosula, S., Nikolopoulou, A.: Radioiodinated
Metaiodobenzylguanidine (MIBG): Radiochemistry,
Biology, and Pharmacology, Semin. Nucl. Med. 41
(2011) 324-33.
Wackers, F. J. T., Berman, D. S., Maddahi, J., Watson, D.
D., Beller, G. A., Strauss, H. W., Boucher, C. A.,
Picard, M., Holman, B. L., Fridrich, ,R., Inglese, E.,
Delaloye, B., Bischofdelaloye, A., Camin, L.,
Mckusick K.: Technetium-99m Hexakis 2-
Methoxyisobutyl Isonitrile: Human Biodistribution,
Dosimetry, Safety, and Preliminary Comparison to
Thallium-201 for Myocardial Perfusion Imaging. J
Nucl Med 30 (1989) 301-311.
Yu, M., Nekolla, S. G., Schwaiger, M., Robinson, S. P.:
The next generation of cardiac positron emission
tomography imaging agents: Discovery of Flurpiridaz
F-18 for detection of coronary disease, Semin. Nucl.
Med. 41 (2011) 305-313.
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
78
