
Motor Unit Properties and Underlying Determinants in Pathological
Tremor
J. A. Gallego
1
, J. L. Dideriksen
2
, A. Holobar
3
, J. P. Romero
4
, J. L. Pons
1
, E. Rocon
1
and D. Farina
2
1
Bioengineering Group, Spanish National Research Council (CSIC), Arganda del Rey, Spain
2
Department of Neurorehabilitation Engineering, Bernstein Focus Neurotechnology G
¨
ottingen,
Bernstein Center for Computational Neuroscience, University Medical Center G
¨
ottingen,
Georg-August University, G
¨
ottingen, Germany
3
Faculty of Electrical Engineering and Computer Science, University of Maribor, Maribor, Slovenia
4
Department of Neurology, University Hospital ‘12 de Octubre’, Madrid, Spain
Keywords:
Motor Unit, Electromyography, Tremor, Essential Tremor, Coherence, Synchronization.
Abstract:
Pathological tremors are accepted to originate from the projection of supraspinal pathological oscillations to
motor neurons that innervate the affected muscles. These oscillations interact with other neural mechanisms
such as reflexes, and, together with the mechanical properties of the limb, determine the characteristics of
the tremor. However, much is yet unknown about the exact mechanisms that mediate the different types of
tremor, and how they interact. Given that the neural drive to muscle encodes information about all pathways
that regulate movement, we are investigating the properties of motor unit activities in tremor patients and
the factors that determine them as a means to further our understanding of the disorder. This paper presents
a simulation study that supports our departure hypothesis (that tremor is a common cortical projection to the
motor neuron pool), and first experimental evidences on a patient with essential tremor. The latter illustrate that
the predictions derived from the model provide significant support for the analysis of real data, and demonstrate
the potential of the analysis techniques here employed.
1 INTRODUCTION
The term pathological tremor encompasses a series of
disorders that originate disabling involuntary oscilla-
tory activity of a body part (Deuschl et al., 1998).
Such oscillatory movement may be ultimately gen-
erated by different mechanisms, such as pathological
oscillations at cortical and subcortical structures–the
underlying cause in most types of tremor–, or a pe-
ripheral neuropathy–a rare type of tremor– (Deuschl
et al., 2001). In addition, due to the intrinsic proper-
ties of the neuromuscular system, other factors such
as the mechanical properties of the limb (muscles)
and spinal and supraspinal afferent loops are also
thought to participate in tremorogenesis (McAuley
and Marsden, 2000).
A motor unit, understood as a motor neuron and
all the fibers it innervates, is the smallest element that
the nervous system can activate (Heckman and Enoka,
2004). The sum of the action potentials fired by all
active spinal motor neurons, which is referred to as
neural drive to muscle, encodes information about de-
scending and afferent neural commands (Farina et al.,
2010). Thus we believe that the elucidation of the
properties of the neural drive to muscle in tremor pa-
tients may help to further what is known about the
pathophysiology of these disorders.
Few studies to date have investigated the specific
properties of motor unit spike trains in tremor pa-
tients. A characteristic consistently reported in those
available was the presence of paired or tripled dis-
charges, which occurred rhythmically with the tremor
bursts (Das Gupta, 1963) (Dietz et al., 1974) (Elek
et al., 1991) (Baker et al., 1992) (Christakos et al.,
2009). Further, although many works have put for-
ward that motor unit spike trains in tremor patients
exhibit higher synchronization than in healthy sub-
jects (Dietz et al., 1974) (McAuley and Marsden,
2000) (Christakos et al., 2009) (Elble and Deuschl,
2009), no study has specifically addressed this obser-
vation. Importantly, all of the previous works were
constrained by the limited number of units concur-
rently identified, typically less than 5–6 per contrac-
tion (Stashuk et al., 2004), a drawback that arises
133
A. Gallego J., L. Dideriksen J., Holobar A., P. Romero J., L. Pons J., Rocon E. and Farina D..
Motor Unit Properties and Underlying Determinants in Pathological Tremor.
DOI: 10.5220/0004679101330138
In Proceedings of the International Congress on Neurotechnology, Electronics and Informatics (DeNeuro-2013), pages 133-138
ISBN: 978-989-8565-80-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

from state of the art technologies for recording in-
tramuscular electromyograms (EMG). To circumvent
this limitation, in our study we employe a novel
technique that decomposes the multichannel surface
EMG into constituent motor unit spike trains. This
technique, called Convolution Kernel Compensation
(CKC) (Holobar and Zazula, 2007) (Holobar et al.,
2009), has been largely employed to identify motor
unit spike trains in healthy subjects (Holobar et al.,
2010), and deals effectively with the specific firing
properties observed in tremor patients (Holobar et al.,
2012).
This work presents an overview of our current
work on the investigation of tremor properties based
on the analysis of motor unit spike trains. We hypoth-
esize that, in tremor patients, the characteristics of
motor unit firing patterns are determined by the pres-
ence of a strong synaptic input related to (i.e at the fre-
quency of) the tremor, which is commonly projected
from supraspinal centers to the entire motor neuron
pool. Thus, here we review a study with a multiscale
model of pathological tremor in which we tested this
departure hypothesis (Gallego et al., 2011), and com-
pare these results with data obtained from one patient
with essential tremor (ET), the most common type of
tremor, and that originates at the cerebellothalamo-
cortical loops (Benito-Le
´
on and Louis, 2006).
2 Methods
2.1 Computational Model
We employed a multiscale model of a pair of antag-
onist muscles to simulate tremor (Dideriksen et al.,
2011). This model comprised a realistic representa-
tion of a motor neuron pool (Fuglevand et al., 1993),
which served to continuously estimate motor unit fir-
ings during dynamic contractions. The net synaptic
input to each motor neuron integrated a descending
voluntary drive and a descending tremor component,
both of which were commonly projected to the entire
motor neuron pool, and afferent input from muscle
spindles and Golgi tendon organs. EMG was simu-
lated employing a model of multilayer cylindrical vol-
ume conductor that comprised anisotropic muscle tis-
sue and isotropic bone (Farina and Merletti, 2004).
Joint dynamics and afferent inputs were calculated
with a model that accounted for the viscoelastic prop-
erties of muscles. The muscles simulated were the
first dorsal interosseus (FDI) and its antagonist, the
second palmar interosseus. Model parameters were
set to those in in (Dideriksen et al., 2011). Simu-
lations were performed at four different contraction
levels (0, 5, 10 and 20 % of the maximum voluntary
contraction [MVC]), and with three different imposed
tremor frequencies (5, 8 and 11 Hz); see (Gallego
et al., 2011) for details.
2.2 Patient and Protocol
We present data for one female ET patient (79 years
old) with bilateral postural and kinetic tremor of mod-
erate severity, recruited at Hospital Universitario “12
de Octubre,” Madrid, Spain. The patient, who was
on medication (propanolol, 120 mg/day) during the
recordings, did not exhibit head or trunk tremor. The
Ethical Committee at the hospital approved the ex-
perimental protocol, and the patient signed a written
informed consent to participate.
The recordings were carried out while the patient
was sitting on an armchair in a dimly illuminated
room. Tremor was triggered by asking the patient to
outstretch both hands, with the palms down and the
fingers slightly outspread, while the forearm was fully
supported. Data were recorded for 4 min.
Hand tremor at the most affected side (right) was
recorded with a multichannel EMG electrode grid (13
x 5 electrodes, 8 mm inter-electrode distance, LISiN–
OT Bioelettronica, Torino, Italy) placed over the ex-
tensor digitorum communis; a moistened bracelet at-
tached to the wrist served as common reference. Con-
currently, we recorded electroencephalographic activ-
ity (EEG) from 32 positions at the somatosensory-
cortex (AFz, F3, F1, Fz, F2, F4, FC5, FC3, FC1,
FCz, FC2, FC4, FC6, C5, C3, C1, Cz, C2, C4, C6,
CP5, CP3, CP1, CPz, CP2, CP4, CP6, P3, P1, Pz, P2,
and P4 according to the 10-20 system) with passive
Au electrodes; the common potential of the two ear-
lobes was used as reference, and Az as ground. EMG
signals were amplified (EMGUSB, OT Bioelettron-
ica, Torino, Italy), band-pass filtered (10–750 Hz),
and sampled at 2,048 Hz by a 12-bit A/D converter;
EEG signals were amplified (gUSBamp, g.Tec gmbh,
Graz, Austria), band-pass (0.1–60 Hz) and notch (50
Hz) filtered, and sampled at 256 Hz by a 16 bit A/D
converter.
2.3 Data Processing and Analysis
The analysis focused on two aspects. First we as-
sessed, with the model, how the tremor drive com-
monly projected from supraspinal centers to the entire
motor neuron pool would be transmitted to the output
of such motor neuron population. We expected that
linear sampling would arise after the spike trains of a
few motor neurons were considered together, as ob-
served for the voluntary drive in healthy subjects (Ne-
NEUROTECHNIX2013-InternationalCongressonNeurotechnology,ElectronicsandInformatics
134
33 11 55 10
0
0.02
0.04
0.06
0.08
0.1
number of motor unitsnumber of motor units
1.05
0.95
0.90
0.85
1
A B
coherence
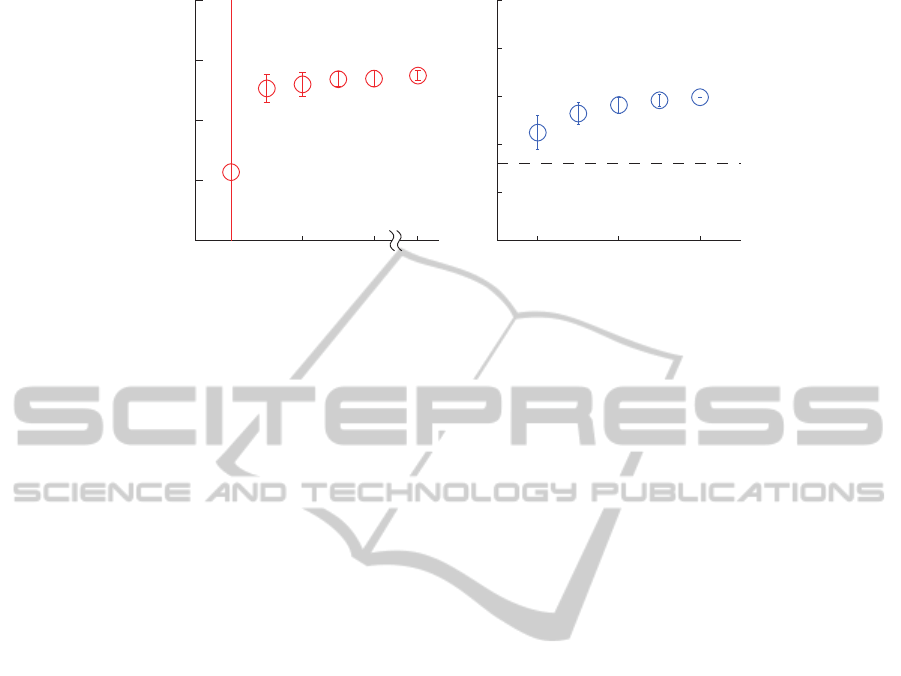
Figure 1: Coherence between the supraspinal tremor oscillations and the CSTs, as function of the number of motor units
considered in the CST. Data in (A) corresponds to the model, in (B) to the patient. The plot represents the mean ± SD for
all possible combinations (in the case of the model considering 1 out of 10 motor units recruited). In (B) the dashed line
represents the significance threshold (P < 0.05); in the case of (A) it is now visible (it was 0.145 for P < 0.05).
gro and Farina, 2011). Then, we investigated whether
the patient data replicated the behavior expected from
the model. In a second phase, we compared qual-
itatively the simulated and observed (in the patient)
properties of motor unit spike trains, in terms of firing
statistics and synchronization between the activities
of different motor units.
Linear sampling of the (supraspinal) tremor in-
put by the motor neuron pool was investigated by
calculating the coherence between groups of motor
unit spike trains (referred to as composite spike trains
[CST]) and the tremor drive. We also assessed how
coherence varied as more motor units were included
in the CST in order to verify the hypothesis that, in
patients, the tremor is a common cortical input to
the motor neuron pool; if the projection were com-
mon, the coherence at the tremor frequency should
increase until reaching a plateau (saturation) for a few
motor units (Negro and Farina, 2011), meaning that
the motor neuron pool had linearized the transmis-
sion of the tremor drive. In the case of the model,
the tremor drive was directly available for the com-
putations, while for the patient it was assessed from
the raw EEG. In both cases, coherence was estimated
in 1-s disjoint Hann windows (Halliday et al., 1995);
in the case of the patient, windows contaminated with
artefacts were carefully removed. Significance was
estimated following (Rosenberg et al., 1989).
Motor unit behavior was assessed by computing
histograms of their inter-spike intervals (ISI), in or-
der to have a global representation of their statis-
tical properties. Special attention was paid to the
presence of paired or tripled discharges (Das Gupta,
1963) (Dietz et al., 1974) (Elek et al., 1991) (Baker
et al., 1992) (Christakos et al., 2009). Further, we
assessed the synchronization between pairs of motor
units to investigate whether synchronization is greater
in tremor patients than in healthy counterparts. In the
model, we expected that abnormally large synchro-
nization would arise due to the presence of a com-
mon synaptic input related to tremor. In the case of
patients, it would be suggestive of the presence of a
strong common input (Kirkwood and Sears, 1978). A
standard metric based on the computation of cross-
correlograms between pairs of motor unit spike trains,
the Common Input Strength index (CIS) (Nordstrom
et al., 1992) was employed. Significant synchroniza-
tion was inferred from the cumulative sum of the
cross-correlogram (Ellaway, 1978). For the calcu-
lation, we followed the methodology in (Dideriksen
et al., 2011) (Gallego et al., 2011).
For the patient, we identified motor unit spike
trains from the decomposition of the multichannel
EMG using the CKC technique (Holobar et al., 2012),
as mentioned above. Motor unit spike trains were ver-
ified by an experienced operator, and motor neurons
that were not active for a significant proportion of the
trial (<65 %) or identified with great accuracy (height
of spike trains compared to baseline jitter ≥26 dB)
were discarded for the analysis. In the case of the
model, motor unit spike trains were directly available.
Results are reported as mean ± SD.
3 RESULTS
For the patient, the total number of identified motor
units that satisfied the criteria presented above were
5. The number of high quality 1-s EEG windows was
93. These data were employed for the subsequent ex-
perimental analyses, except where mentioned other-
wise.
MotorUnitPropertiesandUnderlyingDeterminantsinPathologicalTremor
135
3.1 Common Supraspinal Input
For all simulated conditions (amount of voluntary
contraction and imposed tremor frequency), the co-
herence at the tremor frequency was largely signifi-
cant even when 1 single motor unit was considered
(grand mean for all conditions 0.953 ± 0.027; the
confidence limit was 0.145 for P < 0.05), as expected
from the implementation of the model (Dideriksen
et al., 2011) (Gallego et al., 2011). Even for a such a
large coherence with CSTs comprising 1 motor unit,
when assessing the relationship between coherence
and number of motor units in the CST (see Fig. 1A),
we observed that there was an exponential trend that
reached a plateau when ∼4 motor neurons were con-
sidered (visual inspection). This suggests that the ex-
istence of a common synaptic input related to tremor
could be inferred, even in the concurrent presence of
a voluntary drive, by observing the trend of the co-
herence peak at the tremor frequency as function of
the number of motor units sampled, as previously ob-
served for healthy subjects (Negro and Farina, 2011).
As to the patient, we obtained significant coher-
ence at the tremor frequency (5.750 Hz) between the
contralateral cortical activity (largest at CP3) even
when 1 motor unit was sampled (coherence 0.045;
the confidence limit was 0.032 for P < 0.05). As ex-
pected, the coherence was considerably smaller than
for the model given the presence of interneurons in
the descending pathways, which distorted the trans-
mission process due to their nonlinear transfer func-
tion (Gerstner and Kistler, 2002). Interestingly, the
analysis of corticospinal coherence as function of the
number of motor units also indicated that there was
an exponential trend (see Fig 1B), and thus suggested
that the descending tremor drive was a common input
at the tremor frequency.
3.2 Motor Unit Firing Properties and
Synchronization
In the model, the analysis of motor unit behavior
yielded that motor unit firing characteristics were
largely influenced by motor neuron size (which de-
termines its recruitment threshold (Fuglevand et al.,
1993)) and by the strength of the voluntary drive and
the frequency of the tremor. As motor neuron size in-
creased, at low contraction levels, motor neurons ex-
hibited less likelihood to fire paired and tripled dis-
charges. On the contrary, at strong voluntary con-
tractions small motor units exhibited a firing pattern
that resembled that observed during voluntary con-
tractions in the absence of tremor, and larger motor
neurons fired paired and tripled discharges depend-
0 5 10 20
-2
0
2
4
6
8
10
12
voluntary contraction (% MVC)
CIS (pps)
5 Hz
8 Hz
11 Hz
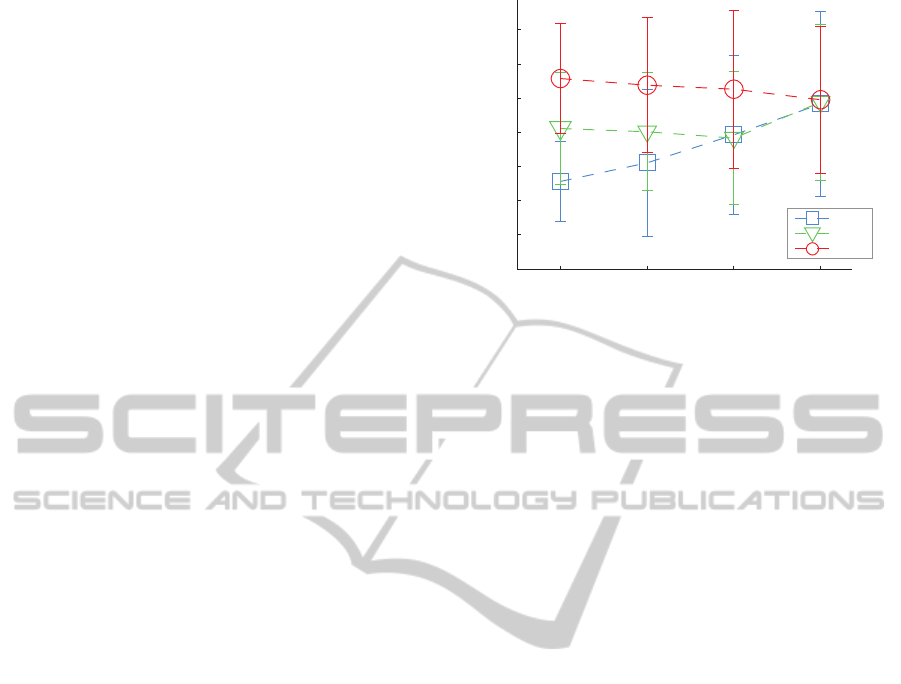
Figure 2: Motor unit synchronization as computed with the
CIS. The plot illustrates the mean ± SD synchronization
for all possible pairs of motor neurons (considering 1 out of
each 10 simulated) for 4 contraction levels and 3 different
tremor frequencies (see the legend).
ing on several factors (such as the strength of the
voluntary drive, tremor frequency, and motor neuron
size). Higher tremor frequencies appeared to facili-
tate the transition from rhythmic motor unit firing (en-
trained with the tremor) to a tonic firing pattern. Ac-
cordingly, the ISI histograms of the simulated motor
unit spike trains followed both unimodal and bimodal
(with one peak reflecting the paired and tripled dis-
charges) distributions. Interestingly, motor unit syn-
chronization as estimated with the CIS was remark-
ably larger (grand mean for all conditions 6.745 ±
1.870 pps) than when simulating voluntary contrac-
tions in the absence of tremor (mean CIS for the 4
contraction levels 0.203 ± 0.159 pps). Fig. 2 illus-
trates the influence of strength of voluntary contrac-
tion and tremor frequency on motor unit synchroniza-
tion as estimated with the CIS.
The patient data followed an unimodal, slightly
skewed distribution with a peak at ∼50 ms (joint data
for the 5 identified motor units, i.e. cumulative ISI
histogram). Unexpectedly, this value was not related
to tremor frequency. However, the motor units often
fired paired and tripled discharges (ISI 20–80 ms), as
previously reported in a study on ET patients (Elek
et al., 1991). Nine out of 10 pairs of motor unit spike
trains exhibited significant correlation, and the mean
CIS for the last 2 min of the trial was 1.61 ± 1.48
pps. This value was remarkably larger than previ-
ously reported for healthy subjects performing vol-
untary contractions (≤0.7 pps (Keen and Fuglevand,
2004)), which indicates that the motor neuron pool
receives a strong common synaptic input.
NEUROTECHNIX2013-InternationalCongressonNeurotechnology,ElectronicsandInformatics
136

4 DISCUSSION
This paper showed, based on a multiscale simulation
model and data on one representative tremor patient,
that the supraspinal oscillations that mediate tremor
are a common cortical projection to the motor neuron
pool, as indirectly suggested in previous works com-
puting EEG-EMG coherence. Furthermore it puts
forward, based on physiologically plausible simula-
tions, that the presence of a common tremor drive to
the motor neuron pool may cause abnormally large
motor unit synchronization, as largely hypothesized
for tremor. Experimental results in one representa-
tive ET patient suggest that this abnormally large syn-
chronization may be systematically found in patients,
which would indirectly prove the existence of a strong
common synaptic input related to tremor.
It must be noticed that the coherence values found
in the model were notably larger than those observed
in the patient. We believe that the reason for this is
(at least) twofold. First, in the model the tremor was a
narrowband colored noise that was directly projected
to all motor neurons in the pool, which implies that it
neglects the spectral distortion that interneurons intro-
duce in the transmission of supraspinal oscillations,
given their nonlinear response (Gerstner and Kistler,
2002) (Negro and Farina, 2011). Second, in the model
the central (supraspinal) tremor was directly avail-
able, while for the case of the patient we had to as-
sess its contribution from the EEG. Indeed, the am-
plitude spectrum of the EEG recorded in this patient
did not shown a peak at the tremor frequency, oppo-
site to the clear contribution observed in the case of
the model. Remarkably, low coherence values are re-
ported in other works studying EEG-EMG coherence
in tremor patients (Raethjen et al., 2007) (Volkmann
et al., 1996) (Timmermann et al., 2003).
As to motor neuron synchronization, for the
model we showed that if a strong common tremor in-
put was projected to the entire motor neuron popula-
tion, the motor unit spike trains exhibited abnormally
synchronization. Interestingly, synchronization was
remarkably large for all tremor frequencies and lev-
els of concurrent voluntary contraction. In the case
of the patient, we observed that motor unit synchro-
nization as computed with the CIS was more than
two times larger than previously reported for healthy
counterparts. Thus, given the well-established causal
relationship between synchronization and common
synaptic inputs (Kirkwood and Sears, 1978) we con-
sider this as a further proof of the existence of a com-
mon cortical input to the motor neuron pool related to
tremor.
ACKNOWLEDGEMENTS
This work has been funded by the EU Commission
through grants EU-FP7-2007-224051 (TREMOR)
and EU-FP7-2011-287739 (NeuroTREMOR).
REFERENCES
Baker, J. R., Davey, N. J., Ellaway, P. H., and Friedland,
C. L. (1992). Short-term synchrony of motor unit dis-
charge during weak isometric contraction in parkin-
son’s disease. Brain, 115 Pt 1:137–154.
Benito-Le
´
on, J. and Louis, E. D. (2006). Essential tremor:
emerging views of a common disorder. Nature clinical
practice. Neurology, 2(12):666–78;.
Christakos, C. N., Erimaki, S., Anagnostou, E., and Anasta-
sopoulos, D. (2009). Tremor-related motor unit firing
in parkinson’s disease: implications for tremor gene-
sis. J Physiol, 587(Pt 20):4811–4827.
Das Gupta, A. (1963). Paired response of motor units dur-
ing voluntary contraction in parkinsonism. J Neurol
Neurosurg Psychiatry, 26:265–268.
Deuschl, G., Bain, P., and Brin, M. (1998). Consensus state-
ment of the movement disorder society on tremor. ad
hoc scientific committee. Mov Disord, 13 Suppl 3:2–
23.
Deuschl, G., Raethjen, J., Lindemann, M., and Krack, P.
(2001). The pathophysiology of tremor. Muscle &
Nerve, 24:716–735.
Dideriksen, J., Enoka, R., and Farina, D. (2011). A model
of the surface electromyogram in pathological tremor.
IEEE Trans Biomed Eng.
Dietz, V., Hillesheimer, W., and Freund, H. J. (1974). Corre-
lation between tremor, voluntary contraction, and fir-
ing pattern of motor units in parkinson’s disease. J
Neurol Neurosurg Psychiatry, 37(8):927–937.
Elble, R. J. and Deuschl, G. (2009). An update on essential
tremor. Neurol Neurosci Reports, 9:273–7.
Elek, J. M., Dengler, R., Konstanzer, A., Hesse, S.,
and Wolf, W. (1991). Mechanical implications of
paired motor unit discharges in pathological and vol-
untary tremor. Electroencephalogr Clin Neurophysiol,
81(4):279–283.
Ellaway, P. H. (1978). Cumulative sum technique and
its application to the analysis of peristimulus time
histograms. Electroencephalogr Clin Neurophysiol,
45(2):302–304.
Farina, D., Holobar, A., Merletti, R., and Enoka, R. M.
(2010). Decoding the neural drive to muscles from
the surface electromyogram. Clin Neurophysiol,
121(10):1616–1623.
Farina, D. and Merletti, R. (2004). Estimation of av-
erage muscle fiber conduction velocity from two-
dimensional surface emg recordings. J Neurosci
Methods, 134(2):199–208.
Fuglevand, A. J., Winter, D. A., and Patla, A. E. (1993).
Models of recruitment and rate coding organization in
motor-unit pools. J Neurophysiol, 70(6):2470–2488.
MotorUnitPropertiesandUnderlyingDeterminantsinPathologicalTremor
137

Gallego, J. A., Dideriksen, J. L., Farina, D., Rocon, E.,
Holobar, A., and Pons, J. L. (2011). A modelling
study on transmission of the central oscillator in
tremor by a motor neuron pool. In Proc. Annual Int
Engineering in Medicine and Biology Society,EMBC
Conf. of the IEEE, pages 2037–2040.
Gerstner, W. and Kistler, W. M. (2002). Spiking neuron
models. Single neurons, populations, plasticity. Cam-
bridge University Press.
Halliday, D. M., Rosenberg, J. R., Amjad, A. M., Breeze,
P., Conway, B. A., and Farmer, S. F. (1995). A frame-
work for the analysis of mixed time series/point pro-
cess data–theory and application to the study of physi-
ological tremor, single motor unit discharges and elec-
tromyograms. Prog Biophys Mol Biol, 64(2-3):237–
278.
Heckman, C. J. and Enoka, R. M. (2004). Handbook of
Clinical Neurophysiology, vol. 4, Clinical Neurophys-
iology of Motor Neuron Diseases, chapter Physiology
of the motor neuron and the motor unit, pages 119–
147. Elsevier.
Holobar, A., Farina, D., Gazzoni, M., Merletti, R., and Za-
zula, D. (2009). Estimating motor unit discharge pat-
terns from high-density surface electromyogram. Clin
Neurophysiol, 120(3):551–562.
Holobar, A., Glaser, V., Gallego, J. A., Dideriksen, J. L., and
Farina, D. (2012). Non-invasive characterization of
motor unit behaviour in pathological tremor. J Neural
Eng, 9(5):056011.
Holobar, A., Minetto, M. A., Botter, A., Negro, F., and Fa-
rina, D. (2010). Experimental analysis of accuracy in
the identification of motor unit spike trains from high-
density surface emg. IEEE Trans Neural Syst Rehabil
Eng, 18(3):221–229.
Holobar, A. and Zazula, D. (2007). Multichannel blind
source separation using convolution kernel compen-
sation. Signal Processing, IEEE Transactions on,
55(9):4487 –4496.
Keen, D. A. and Fuglevand, A. J. (2004). Common input
to motor neurons innervating the same and different
compartments of the human extensor digitorum mus-
cle. J Neurophysiol, 91(1):57–62.
Kirkwood, P. A. and Sears, T. A. (1978). The synaptic con-
nexions to intercostal motoneurones as revealed by
the average common excitation potential. J Physiol,
275:103–134.
McAuley, J. H. and Marsden, C. D. (2000). Physiological
and pathological tremors and rhythmic central motor
control. Brain, 123:1545–1567.
Negro, F. and Farina, D. (2011). Linear transmission of cor-
tical oscillations to the neural drive to muscles is me-
diated by common projections to populations of mo-
toneurons in humans. J Physiol, 589(Pt 3):629–637.
Nordstrom, M. A., Fuglevand, A. J., and Enoka, R. M.
(1992). Estimating the strength of common input to
human motoneurons from the cross-correlogram. J
Physiol, 453:547–574.
Raethjen, J., Govindan, R. B., Kopper, F., Muthuraman,
M., and Deuschl, G. (2007). Cortical involvement
in the generation of essential tremor. J Neurophysiol,
97(5):3219–3228.
Rosenberg, J. R., Amjad, A. M., Breeze, P., Brillinger,
D. R., and Halliday, D. M. (1989). The fourier ap-
proach to the identification of functional coupling be-
tween neuronal spike trains. Prog Biophys Mol Biol,
53(1):1–31.
Stashuk, D. W., Farina, D., and Sgaard, K. (2004). Elec-
tromyography: Physiology, Engineering, and Nonin-
vasive Applications, chapter Decomposition of intra-
muscular emg signals. Wiley–IEEE Press.
Timmermann, L., Gross, J., Dirks, M., J., V., H.J., F., and
Schnitzler, A. (2003). The cerebral oscillatory net-
work of parkinsonian resting tremor. Brain, 126:199–
212.
Volkmann, J., Joliot, M., Mogilner, A., Ioannides, A. A.,
Lado, F., Fazzini, E., Ribary, U., and Llins, R. (1996).
Central motor loop oscillations in parkinsonian resting
tremor revealed by magnetoencephalography. Neurol-
ogy, 46(5):1359–1370.
NEUROTECHNIX2013-InternationalCongressonNeurotechnology,ElectronicsandInformatics
138
