
High Efficiency and Low Photodegradation in Random Laser, using
Novel TiO
2
@Silica Nanoparticles
Ernesto Jimenez-Villar
1,2
, Valdeci Mestre
3
, Paulo C. De Oliveira
3
,
Wagner M. Faustino
4
and Gilberto F. De Sá
1
1
Departamento de Química Fundamental, Universidade Federal de Pernambuco, Recife, PE,50670-901, Brazil
2
Instituto de Ciencia Molecular, Universitat de València. C/ Catedrático José Beltrán Nº 2, 46980 Paterna Valencia, Spain
3
Departamento de Física, Universidade Federal da Paraíba, João Pessoa, Paraíba 580051-970, Brazil
4
Departamento de Química, Universidade Federal da Paraíba, João Pessoa, Paraíba 58051-970, Brazil
Keywords: Core-Shell Nanoparticles, Random Laser, TiO
2
@Silica Nanoparticles, TiO
2
Dye Photodegradation.
Abstract: Here we have studied a novel scattering medium for random laser. This medium is composed of
TiO
2
@Silica nanoparticles suspended in an ethanol solution of rhodamine 6G. TiO
2
nanoparticles with
average diameter of 0.41 μm were coated with a silica shell of ~40 nm thickness. Random laser study
comparing TiO
2
and TiO
2
@Silica nanoparticles suspended in ethanol solution of rhodamine 6G was
performed. The study showed a high efficiency, low threshold, narrower bandwidth and lower
photodegradation for TiO
2
@Silica system. Optical and chemical stability has been combined by coating
TiO
2
nanoparticles with a silica shell of ~40nm thickness.
1 INTRODUCTION
The first evidence of random laser (RL) in solution
was obtained by Lawandy et. al. (Lawandy, 1994)
who suspended TiO
2
nanoparticles (Np) in a
conventional laser dye. RL action have been
observed in a variety of gain media including
polymeric films with and without intentionally
introduced scatterers (Polson, 2001), in GaN
nanocolumns (Masaru, 2010), dye-infiltrated opals
(Shkunov, 2001), porous media infiltrated with
liquid crystals with dyes (Wiersma, 2001), porous
network of air into a solid glass or semiconductor
crystal (Schuurmans, 1999), ZnO scattering films
and nanoclusters (Cao, 2001), on waveguided
plasmonic (Tianrui, 2011) and many others. In the
works reported by Noginov (Noginov, 2005), Cao
(Cao, 2005) and Wiersma (Wiersma, 2008) detailed
reviews on RL can be found.
The strategy introduced by Lawandy, suspending
highly scattering particles in laser dye has been
repeated by other authors (Noginov, 1995),
(Leonetti, 2012) in order to study the random laser.
However, the photodegradation effect and the
inability to ensure complete colloidal dispersion,
have limited the development and applications of
such systems. The complete colloidal dispersion is
related to an increase of the scattering surface per
unit volume with the suspended particles
concentration. This is extremely difficult to obtain in
solution at high concentrations, because particles
tend to agglomerate (Mandzy, 2005). The surface
modification of TiO
2
Np with a silane coupling
agent has been used in order to reduce the
agglomeration effect and improve the mechanical
properties and UV protection of urethane clear
coatings in TiO
2
composites (Sabzi, 2009). Other
authors have reported the replacement of the
dispersive medium (TiO
2
Np) by silica Np (Brito-
Silva, 2010), demonstrating random lasing. This
kind of scattering medium greatly decreases the
photodegradation effect. However, the relatively
small difference in refractive index between silica
and the alcohol-dye solution in comparison to TiO
2
causes a threshold increase and an efficiency
decrease of the RL. In this work, we propose to
study photodegradation effect and action of RL
composed of TiO
2
@Silica
particles suspended in
ethanol solution of rhodamine 6G (R6G). Particles
like TiO
2
@SiO
2
have already been synthesized
before (over ten years back) (Joseph, 2000),
however, their application in RL has been done very
recently (Jimenez-Villar, 2013), (B-Jimenez-Villar,
26
Jimenez-Villar E., Mestre V., C. De Oliveira P., M. Faustino W. and F. De Sá G..
High Efficiency and Low Photodegradation in Random Laser, using Novel TiO2@Silica Nanoparticles.
DOI: 10.5220/0004711700260032
In Proceedings of 2nd International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS-2014), pages 26-32
ISBN: 978-989-758-008-6
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

2013). In this work, we have studied the RL action
and Photodegradation effect for an extended range
of pumping energy fluencies (between 0.12 and 264
mJ/cm
2
).
The silica shell with thickness around 40 nm
presents a steric effect, preventing the “optical”
junction of scattering TiO
2
surfaces. Moreover, this
silica shell should improve the light coupling with
the TiO
2
particles by light refraction at the ethanol-
silica interface. In addition, silica shell acts as a
barrier to prevent the charge transfer, which is the
principal cause of the dye degradation (Fox, 1993).
These have been practical difficulties for the
development of RL and novel optical devices with
improved performance and functionality. In turn, the
silica coating is particularly advantageous due to its
high dispersibility (Jimenez, 2008), (Jimenez, 2010),
low density, and the inertness of nanoparticles
(Fuertes, 2011), (B-Fuertes, 2011) along with the
numerous possibilities for their use, (Rodriguez,
2005), (Rodriguez, 2008).
Therefore strongly scattering particles coated
with a shell of thickness and refractive index
suitable could open new opportunities to achieve
significant improvements in the operation of RL and
photonic devices based on highly disordered
scattering media.
2 EXPERIMENTAL SECTION
2.1 Chemical Synthesis and
Characterization
Rhodamine 6G laser dye (C
28
H
31
N
2
O
3
Cl) with
molecular weight 479.02 g/mol supplied by Fluka:
Ethanol alcohol (C
2
H
5
OH) with spectroscopic grade
purity supplied by Alphatec: Tetra-ethyl-ortho-
silicate (TEOS) supplied by Sigma-Aldrich.
Titanium dioxide (TiO
2
Np; diameter 410 nm) of
rutile crystal structure was acquired from DuPont
Inc (R900).
Two kinds of samples were prepared containing
[1x10
-4
M] of Rhodamine 6G (R6G), one with TiO
2
and another with TiO
2
@Silica scatters Np. The silica
coating of TiO
2
Np was made via Stöber method
(
Stöber, 1968), (Sheng-Li, 1997), (Abderrafi, 2012).
In
the first stage 2 g of TiO
2
Np were dispersed in 250
ml of absolute ethanol by ultrasound bath for 20
minutes. Then, the solution of TiO
2
Np was divided
into two equal portions of 125 ml. One of the parts
was placed in a bath at 5 °C and 1.1 ml of TEOS,
previously diluted in 11 ml of ethanol, was added.
The 10% diluted solution of TEOS was added in 110
portions of 100 μl during the course of 1 hour. The
solution was stirred during the TEOS addition and
after it was stored during 4 week at room
temperature. The other portion was stored and used
as a reference in every experiment.
The silica coating on the TiO
2
Np were examined
by transmission electron microscopy (TEM),
performed on a 100 kV JEOL, model 1200EX,
microscope. The commercial carbon-coated Cu
TEM grid was immersed in the solution of
TiO
2
@Silica Np previously diluted 50-fold lower
and then left to dry before being introduced into the
microscope. The stoichiometric ratio (Ti/Si) of
nanoparticles (TiO
2
@Silica) was determined by
Energy Dispersive X-Ray fluorescence (ED-XRF)
using an X-ray spectrometer SIEMENS D5000. The
sample was prepared in three steps; precipitation,
washing and drying. The nanoparticles powder
(TiO
2
@Silica) was pressed into a tablet form of a
12mm diameter for analysis.
2.2 Experimental Setup of Random
Laser
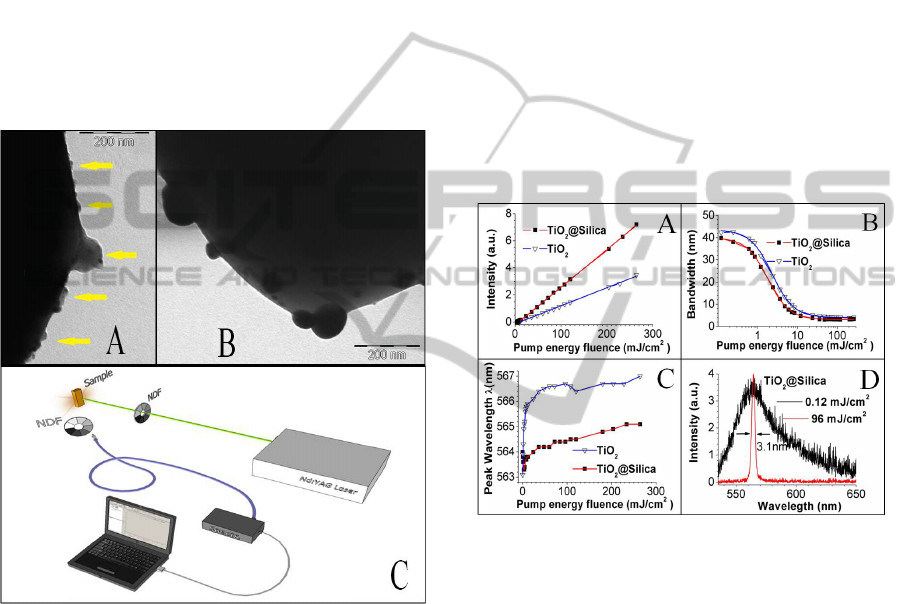
Figure 1C shows a schematic diagram of the RL
experimental setup. The pumping source was the
second harmonic of a Q-switched Nd: YAG
Continuum Minilite II (25 mJ, λ = 532 nm, with a
pulse width of ~6 ns, repetition rate up to 15 Hz, and
spot size of 3 mm). The laser power was regulated
through neutral density filters (NDF), a polarizer and
a half wave plate. The samples were accommodated
in a 2 mm pathlength quartz cuvette. The pump laser
beam was incident upon the sample at 15 deg. The
emission spectra were collected through a
multimode optical fiber (200 μm) coupled to a
spectrometer HR4000 UV-VIS (Ocean Optics) with
0.36 nm spectral resolution (FWHM). The collection
angle (optical fiber) was ~45 deg with respect to the
incident pumping beam, that is, 60 deg with respect
to the cuvette surface. The liquid samples were
placed in an ultrasound bath for about 10 minutes
before recording the spectrum, in order to obtain the
same dispersion of nanoparticles (initial conditions)
in all measurements.
3 RESULTS AND DISCUSSION
3.1 Silica Shell onto TiO
2
Nanoparticles
In TEM images (Figure 1A) we observe the silica
coating on TiO
2
Np, such as the one indicated by the
HighEfficiencyandLowPhotodegradationinRandomLaser,usingNovelTiO2@SilicaNanoparticles
27
yellow arrows. This silica shell presents an irregular
morphology with a thickness ranging between 20 nm
and 70 nm. Figure 1B shows the surface of one TiO
2
Np, before the coating with silica. As can be seen,
the Np surface is irregular; this fact should
determine the morphology of the silica coating
subsequently. The mass percentage ratio (Ti/Si)
determined by ED-XRF was Ti
70
/Si
30
. The average
thickness of silica coating, calculated from the
typical silica density obtained by the TEOS
hydrolysis 2.1 g/cm
3
(Karmakar, 2000), was ~40 nm.
In this way, the silica shell represents a barrier that
prevents the “optical” binding of TiO
2
scattering
surfaces, with the additional advantage to present a
chemically stable surface (SiO
2
).
Figure 1: TEM images of; A) silica coating on the
TiO
2
@Silica surface and B) TiO
2
nanoparticle surface.
The scale bars represent 200 nm. Yellow arrows (A)
indicate the silica coating. C) Schematic diagram of the
RL experimental setup.
3.2 Random Laser Action
Figures 2A and 2B show the behaviour of the
emitted intensity and the spectral width (FWHM), as
a function of pumping energy fluencies for the two
kind of scattering medium (TiO
2
and TiO
2
@Silica).
The RL action for pumping energy fluencies
between 0.12 and 264 mJ/cm
2
were performed. The
calculated concentrations of scatters Np and dye
were 5.6 x10
10
Np/ml and 1x10
-4
M, respectively.
Each value of emission intensity and bandwidth
represented in the graphs (fig. 2A and B) was taken
by integrating 10 laser pulses, which allowed us to
rule out any photodegradation effects during the
measurement. As observed, the RL action for
TiO
2
@Silica system is improved, i.e. presented
higher slope efficiency, narrower bandwidth and
lower laser threshold. For the TiO
2
@Silica system,
the laser slope efficiency was ~2.1 times greater than
for TiO
2
.
The RL threshold values extracted from the
fittings (fig.2B) for TiO
2
and TiO
2
@Silica systems
were 2.29 ±0.04 mJ/cm
2
and 1.79 ±0.02 mJ/cm
2
,
respectively. The highest gain narrowing factor,
defined as the FWHM of the emitted light below
threshold divided by the FWHM of the emission
spectrum of the RL far above threshold gave a value
of 12.2 for TiO
2
@Silica, and 10.6 for TiO
2
, which
corresponds to -factors (
Gijs van Soest, 2002) of
0.082 e 0.094 respectively.
Figure 2: A) The emitted peak intensity and B) spectral
FWHM emission of the RL, for the two kinds of
nanoparticles (TiO
2
and TiO
2
@Silica). The solid lines
represent the fits with experiments points; blue and red
lines correspond to the TiO
2
and TiO
2
@Silica systems,
respectively. C) Influence of the pump energy fluence on
peak wavelength of emission spectrums for TiO
2
and
TiO
2
@Silica systems. D) (Black online) Emission spectra
below (broad band spectrum) and above (narrow band
spectrum) the RL threshold for TiO
2
@Silica system.
The peak position of the emission spectrum was
measured as a function of the pumping energy
fluence (between 0.12 and 260 mJ/cm
2
). Figure 2c
shows a comparison of these peak positions with
fluence for the TiO
2
@Silica and TiO
2
systems. The
emission spectrum shows a redshift for the TiO
2
system, which undergoes a large increase in
fluencies between 0.12 and 12 mJ/cm
2
(0 to 2.8 nm).
This redshift increases (between 3 and 3.9 nm) for
fluencies >12 mJ/cm
2
. This shift was previously
observed and explained by a model considering
PHOTOPTICS2014-InternationalConferenceonPhotonics,OpticsandLaserTechnology
28
absorption and emission at the transition between the
ground and the first excited singlet of the dye
molecule (Noginov. 1995). Instead, the emission
spectrum peak for TiO
2
@Silica system shows a
blueshift for fluencies ≤12 mJ/cm
2
. For fluencies
between 12 mJ/cm
2
and 260 mJ/cm
2
, the redshift
increases in the same fashion, from 0 up to ~1 nm. A
comparison between the emission spectra of the
TiO
2
@Silica system for fluencies well below (0.12
mJ/cm
2
) and far above RL threshold (96 mJ/cm
2
) is
showed in the figures 2D. The peaks intensities of
the narrow and broad bands were normalized to
show the narrowing effect more clearly. The peak
intensity relationship (narrow/broad) is ~4 orders
magnitude larger. The redshift of the RL spectrum is
almost null (<0.5nm) at this fluence (96 mJ/cm
2
).
This effect should be due to the fact that the ratio
between R6G molecules and R6G molecules
involved in the stimulated emission is close to unit
[R6G]/[R6G
stimulated
]≈1 at 96mJ/cm
2
, which is
evidenced in a higher efficiency of the RL
(TiO
2
@Silica). The above results could be explained
by the increase of effective scattering surface per
unit volume due to the “optical” colloidal stability
and light coupling enhancement with TiO
2
scattering
cores provided by the silica shell. It is known that
silica Np have a higher colloidal stability than those
of TiO
2
(Yang. 2008), (Chih-ping, 2010). In this
way, the scattering mean free path (l
s
) should be
lower for TiO
2
@Silica system, which mean that
pumping energy is confined in a lower volume.
Furthermore, the amount of R6G molecules inside
the excited volume is lower, being able to excite a
higher percentage of molecules. The scattering mean
free path measured for TiO
2
and TiO
2
@Silica
systems were 52 ±4μm and 20.6 ±0.2μm,
respectively (Jimenez-Villar, 2013). Notice that, the
volume of emission laser should increase with
pumping fluence (I
P0
). The pumping fluence at a
depth length l inside the scattering medium (I
Pl
)
could be expressed as follows:
(1)
l
a
is the ballistic absorption length. The diffuse
intensity has been neglected. When
≪
,
(2)
Therefore, there would be a limit depth length (l
T
)
inside the scattering medium, beyond which the
pumping intensity (I
PT
) is unable to provoke
population inversion. The l
T
should depend on the
pumping fluence I
P0
as follows:
→
∗
ln
ln
(3)
I
PT
would correspond with the RL threshold fluence.
Therefore, for
≫
then l
T
is directly
proportional to l
s
. In turn, the effective pumping
intensity into the RL emission region is inversely
proportional to l
s
, so, it should be higher for
TiO
2
@Silica system.
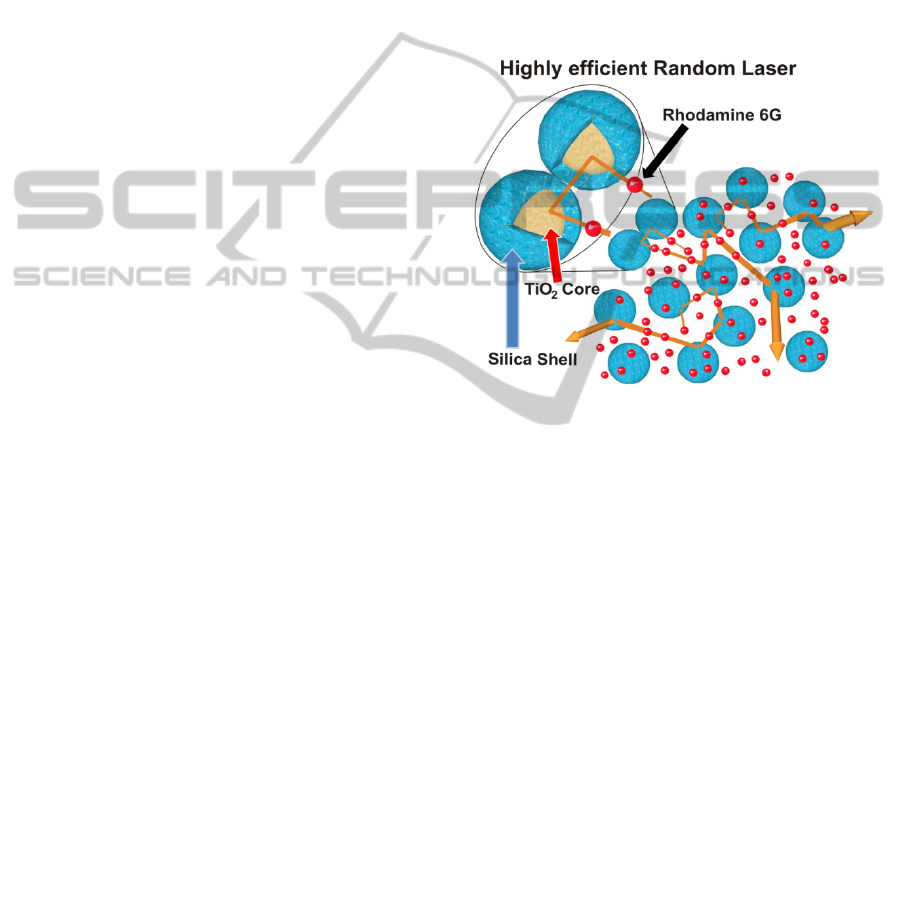
Figure 3 shows a RL representative scheme
consisting of a TiO
2
@Silica Nps suspension in an
ethanol solution of R6G. The silica shell avoids the
contact between TiO
2
scattering surfaces, leading to
a scattering area per unit volume higher and
consequently to an increase of scattering strength.
Figure 3: Representative scheme of the random laser, it
consists of a TiO
2
@Silica Nps suspension in an ethanol
solution of R6G. The blue coating represents the silica
shell on the TiO
2
Nps and the little red spheres correspond
to the R6G molecules. The silica shells between two TiO
2
cores lead to a scattering strength increasing.
3.3 Photodegradation Study
Figure 4 shows the photodegradation process by the
RL emission intensity as a function of shots number
for systems TiO
2
(A) and TiO
2
@Silica (B). The
laser beam of 3 mm diameter and fluencies of 200
mJ/cm
2
and 260 mJ/cm
2
, was used to pump the
samples, which volume was 200 μl accommodated
in a 2 mm pathlength quartz cuvette. Fig. 3A and 3B
show a decrease in emission intensity (RL) with the
number of shots for the pumping fluencies 200
mJ/cm
2
(red) and 260 mJ/cm
2
(black). The TiO
2
system shows a rapid exponential decay. The
number of shots for which the emission intensity
decreases to 50% for the fluencies of 200 and 260
mJ/cm
2
was 960 and 342, respectively. However, for
the TiO
2
@Silica system the number of shots
required were much higher, 59077 (200 mJ/cm
2
) and
26010 (260 mJ/cm
2
), respectively. These represent a
decrease in the photodegradation rate more than 60
HighEfficiencyandLowPhotodegradationinRandomLaser,usingNovelTiO2@SilicaNanoparticles
29
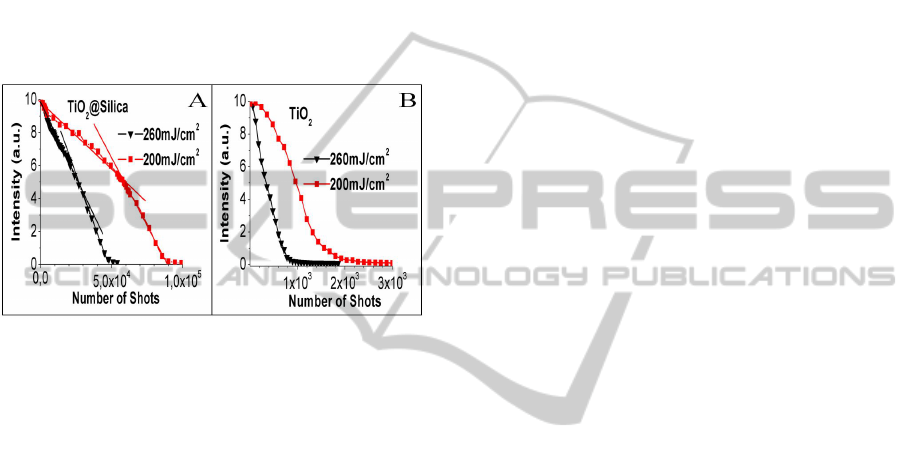
times (200 mJ/cm
2
) and 74 times (260 mJ/cm
2
),
respectively.
The TiO
2
photocatalytic properties are a well
studied subject, which has been used to remove or
degrade dyes from the environment (G. van, 1993).
The photocatalytic pathway involves a reaction on
the TiO
2
surface following several steps: 1)
photogeneration of electron–hole pairs by exciting
the semiconductor with >3.2 eV light; 2) separation
of electrons and holes by traps existing on the TiO
2
surface; 3) a redox process induced by the separated
electrons and holes with the adsorbates present on
the surface.
Figure 4: A-B) Photodegradation process of Random laser
action as a function of the number of shots for the laser
pumping fluencies of 200 mJ/cm
2
(red) and 260 mJ/cm
2
(black): A) TiO
2
Np system; B) TiO
2
@Silica Np system.
The exponential decrease of the RL intensity, for the
system TiO
2
, indicates that the photodegradation is
proportional to its derivative, as to the
photodegradation rate. This means that the charge
transfers (Amy, 1995) and therefore the redox
reaction (Serpone, 1989) will cause a greater charge
transfer in the next laser shot. Thus, one might think
that the high concentrations of charges created by
the TiO
2
nanoparticles at high pumping fluencies
must react with the proper surface of the
nanoparticles, reducing Ti
4+
and oxidizing O
2-
. This
process results in oxygen vacancies (Tsukamoto,
2008), which act as traps for photoelectrons. These
electrons, trapped near the surface, act as a source of
electron transfer coming from these superficial traps,
increasing the efficiency of the redox process
(Heinz, 1991). Additionally, the creation of oxygen
vacancies in TiO
2
causes a progressive decreasing of
gap on the nanoparticle surfaces (TiO
2
), which is
reflected in the progressive increase in the creation
of electron-hole pairs. This photo-darkening effect is
observed in films of TiO
2
exposed to successive
irradiation of laser pulses (Tsukamoto, 2011),
(Tsukamoto, 2008).
The photodegradation process for the TiO
2
@Silica
system presents a linear behaviour. However, the
modulus of the slope increases slightly after the
emission intensity decreases to 50%. Subsequently,
the photodegradation rate experiences a slight
increase, but remains constant. This phenomenon
could be due to the decreased absorption of R6G,
provoking an increase of the effective pumping
fluence inside the scattering medium (TiO
2
@Silica),
which should increase the photodegradation rate of
R6G.
The photodegradation process (RL) for the
system TiO
2
@Silica could be explained through the
reaction of the ethanol radical CH
3
CHOH with R6G
ground state molecules (Adrian, 1976). The free
radical CH
3
CHOH is produced by energy transfer
from the R6G molecules in a higher triplet state,
which is produced by two sequential single-photon
absorptions (Yamashita, 1976). This
photodegradation process is much less effective,
since it does not involve charges transfer from the
TiO
2
nanoparticles, which is known as an efficient
photocatalyzers.
4 CONCLUSIONS
The RL action using a novel scattering media
composed by titanium oxide Np coated with ~40nm
thickness of silica shell was studied. This scattering
medium (TiO
2
@Silica Np) combine the high
refractive index of TiO
2
with chemical inertness,
“optical” colloidal stability and light coupling
enhancement (TiO
2
cores) provided by the silica
shell. Random lasing with higher efficiency, lower
threshold, narrower bandwidth and very long photo-
bleaching lifetime was obtained. The RL efficiency
was 2.1 times higher and the R6G photodegradation
period was between 60 and 74 times higher than the
conventional scattering medium (TiO
2
). The high
RL efficiency was ascribed to lower l
s
for
TiO
2
@Silica system. This fact is associated with the
silica shell, which avoid the “optical” junction of
TiO
2
scattering surface and improves the light
coupling with TiO
2
cores. In other words, the core-
shell scattering particles present a core with high
refractive index (TiO
2
) and a shell that combines
high chemical stability, light coupling enhancement
(TiO
2
cores) and a steric “optical” effect. Therefore,
a lower l
s
provokes higher effective pumping
fluence, leading to higher population inversion and
stimulated emission rate. In this way, it has been
remarked the colloidal stability of the scattering
PHOTOPTICS2014-InternationalConferenceonPhotonics,OpticsandLaserTechnology
30

medium, which is an important parameter and is not
insignificant for the treatment of RL.
The lower photodegradation period was
associated to the higher chemical stability provided
by the silica shell, which should establish a potential
barrier for the charge transfer.
ACKNOWLEDGEMENTS
We gratefully acknowledge financial support from
Rede 36 Nanobiotec CAPES (Brazil). V.M. thanks
the CAPES (Brazil) for doctoral fellowships.
REFERENCES
Lawandy, N. M., Balachandran, R. M., Gomes, A. S. L. &
Sauvain, E., 1994. Nature, 368, 436–438.
Polson R. C., Chipoline A. and Vardeny Z. V., 2001.
Advanced Materials, 13, 760-764.
Masaru Sakai, Yuta Inose, Kazuhiro Ema, Tomi Ohtsuki,
Hiroto Sekiguchi, Akihiko Kikuchi, and Katsumi
Kishino, 2010. Appl. Phys. Lett., 97, 151109.
M. N. Shkunov, M. C. DeLong, M. E. Raikh, Z. V.
Vardeny, A. A. Zakhidov and R. H. Baughman,
Synthetic Metals, 2001, 116, 485-491.
Wiersma D. and Cavalier S., 2001. Nature, 414, 708-709.
Schuurmans, F. J. P., Vanmaekelbergh, D., van de
Lagemaat, J. & Lagendijk, A. 1999. Science, 284,
141–143.
Cao H., Ling Y., Xu J. Y., Cao C. Q. and Kumar P., 2001.
Phys. Rev. Lett., 86, 4524-4527.
Tianrui Zhai, Xinping Zhang, Zhaoguang Pang, Xueqiong
Su, Hongmei Liu, Shengfei Feng, and Li Wang, 2011.
Nano Lett., 11 (10), 4295–4298.
Noginov M. A., 2005. “Solid-State Random Lasers,”
(Springer Series in Optical Sciences), Norfolk State
University, Norfolk.
Hui Cao, 2005. J. Phys. A: Math. Gen., 38, 10497–10535.
Wiersma D. S., 2008. Nature Physics, 4, 359-367.
Sha W., Liu C.-H., and Alfano R., 1994. J.Opt. Soc.
Amer., B 19, 1922–1924.
Noginov M. A., Caulfield H. J., Noginova N. E., and
Venkateswarlu P., 1995. Opt. Commun., 118, 430–
437.
Leonetti M., Conti C. and Cefe Lopez, 2012. Appl. Phys.
Lett., 101, 051104.
Mandzy N., Grulke E., Druffel T., 2005. Powder
Technology, 160, 121–126.
Sabzi M., Mirabedini S.M., Zohuriaan-Mehr J., Atai M.,
2009. Progress in Organic Coatings, 65, 222–228.
Brito-Silva A. M., André Galembeck, Anderson S. L.
Gomes, Alcenisio Jesus-Silva J., Cid B. de Araújo,
2010. Journal of Applied Physics, 108, 033508.
Joseph N. Ryan , Menachem Elimelech , Jenny L.
Baeseman , Robin D. Magelky, 2000. Environ. Sci.
Technol., 34, 2000-2005.
E. Jimenez-Villar, Valdeci Mestre, Paulo C. de Oliveira,
Gilberto F. de Sá, 2013. Nanoscale, in press;
DOI:10.1039/C3NR03603K.
B-E. Jimenez-Villar, Valdeci Mestre, Paulo C. de Oliveira,
Wagner M. Faustino, D. S. Silva, Gilberto F. de Sá,
2013, Appl. Phys, Lett., Submitted.
Fox M. A., Dulay M. T., 1993. Chem. Rev., 93, 341.
E. Jimenez, Kamal Abderrafi, Juan Martınez-Pastor,
Rafael Abargues, Jose Luıs Valdes, Rafael Ibañez,
2008. Superlattices and Microstructures, 43, 487–493.
Ernesto Jimenez, Kamal Abderrafi, Rafael Abargues, Jose
L. Valdes, Juan P. Martınez-Pastor, 2010. Langmuir,
26 (10), 7458–7463.
Gustavo Fuertes, Orlando L. Sanchez-Munoz, Esteban
Pedrueza, Kamal Abderrafi, Jesus Salgado, Ernesto
Jimenez, 2011. Langmuir, 27, 2826–2833.
B-Gustavo Fuertes, Esteban Pedrueza, Kamal Abderrafi,
Rafael Abargues, Orlando Sánchez, Juan Martínez-
Pastor, Jesús Salgado, Ernesto Jiménez, 2011. Proc.
SPIE Medical Laser Applications and Laser-Tissue
Interactions V, No. 8092-51.
Rodriguez E., Jimenez E., Jacob G. J., Neves A. A. R.,
Cesar C. L., Barbosa
L. C., 2005. Physica E, 26, 361.
Rodriquez E., Kellerman G., Jiménez E., César C. L.,
Barbosa L. C., 2008. Superlattices and
Microstructures, 43 (5-6), 626-634.
Stöber W., Fink A., and Bohn E., 1968. J. Colloid
Interface Sci.,26, 62.
Sheng-Li Chen, Peng Dong, Guang-Hua Yang, 1997.
Journal of Colloid and Interface Sci., 189 (2), 268–
272.
Abderrafi Kamal, Jimenez Ernesto, Ben T., Molina S. I.,
Ibáñez R., Chirvony V., Martínez-Pastor J. P., 2012. J.
Nanoscience and Nanotechnology, 12 (8), 6774-6778.
Basudeb Karmakar, Goutam De, Dibyendu Ganguli, 2000.
Journal of Non-Crystalline Solids, 272, 119–126.
Gijs van Soest and Ad Lagendijk, 2002. Physical Review
E, 65, 047601.
Noginov M. A., Caulfield H. J., Noginova N. E.,
Venkateswarlu P., 1995. Optics Communications, 118,
430-437.
Yang Zhang, Yongsheng Chen, Paul Westerhoff, Kiril
Hristovski, John C Crittenden, 2008. Water Research,
42 (8-9), 2204–2212.
Chih-ping Tso, Cheng-min Zhung, Yang-hsin Shih,
Young-Ming Tseng, Shian-chee Wu and Ruey-an
Doong, 2010. Water Science & Technology, 61 (1),
127-133.
van G., Wold A., 1993. Chem. Mater., 5, 280.
Amy L. Linsebigler, Guangquan Lu, and John T. Yates,
1995. Jr. Chem. Rev., 95, 735-758.
Serpone, N., Pelizzetti, E., 1989. “Photocatalysis-
Fundamentals and Applications”, Eds.; Wiley: New
York Chapter 7, van Damme, H. Supports in
Photocatalysis.
Tsukamoto M., Abe N., Soga Y., Yoshida M., Nakano H.,
Fujita M., Akedo J., 2008. Appl Phys A, 93, 193–196.
HighEfficiencyandLowPhotodegradationinRandomLaser,usingNovelTiO2@SilicaNanoparticles
31

Heinz Ceriscber and Adam Heller, 1991. The Journal of
Physical Chemistry, 95 (13), 5261.
Tsukamoto Masahiro, Shinonaga Togo, Takahashi
masanari, Fujita Masayuki,· Abe Nubuyuki, 2011.
Transactions of JWRI, 40 (1), 21-23.
Tsukamoto M., Abe N., Soga Y., Yoshida M., Nakano H.,
Fujita M., Akedo J., 2008. Appl Phys A, 93, 193–196.
Adrian Dunne and Michael F. Quinn, 1976. J. Chem. Soc.,
Faraday Trans. 1, 72, 2289-2295.
Yamashita, M.; Kashiwagi, H., 1976. IEEE Journal of
Quantum electronics, 12 (1) Part:1, 90-95.
PHOTOPTICS2014-InternationalConferenceonPhotonics,OpticsandLaserTechnology
32
