
Data based Modelling of Expired Airflow Clarifies Chronic
Obstructive Pulmonary Disease
Topalovic Marko
1
, Vasileios Exadaktylos
2
, Jean-Marie Aerts
2
, Thierry Troosters
1,3
, Marc Decramer
1
,
Daniel Berckmans
2
and Wim Janssens
1
1
Respiratory Division, University Hospital Leuven, Department of Clinical and Experimental Medicine,
KU Leuven, Leuven, Belgium
2
Measure, Model & Manage Bioresponses (M3 BIORES), Department of Biosystems, KU Leuven, Leuven, Belgium
3
Department of Rehabilitation Sciences, Faculty of Kinesiology and Rehabilitation Sciences, KU Leuven, Leuven, Belgium
Keywords: Data Based Modelling, Transfer Function, Chronic Obstructive Pulmonary Disease, Spirometry, Forced
Expiration.
Abstract: One of the major health challenges of the future is Chronic Obstructive Pulmonary Disease (COPD). It is
characterized by airflow limitations, although current diagnosis does not give attention to the flow
measurements. We aimed to develop a data-based model of the decline of the forced expiratory flow.
Moreover, we analysed the relationship of model parameters with COPD presence and its severity. The
data-based model was developed in 474 smoking individuals, who are at risk of having COPD, and have
performed complete pulmonary function tests in order to identify whether the disease is present and at
which stage. The time series of the decline of the flow was parameterised using the poles and steady state
gain (SSG) of a second order transfer function model. These parameters were then linked with the presence
of COPD. Observing SSG, median (IQR) in subjects with COPD was lower 3.9(2.7-5.6) compared to
8.2(7.1-9.3) in subjects without, (p<0.0001). Significant difference was also found when observing median
(IQR) of two poles in subjects without disease were 0.9868(0.9810-0.9892) and 0.9333(0.9010-0.9529),
respectively, compared to 0.9929(0.9901-0.9952) and 0.9082(0.8669-0.9398) in subjects with COPD
(p<0.001 for both poles). Forced exhaled air can be used to expand understanding of the COPD. Moreover,
the suggested parameterisation of the flow decline could be used to access COPD using spirometry.
1 INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is
one of the major health challenges of the next
decades. Currently it is 4th leading cause of death,
while the World Health Organization anticipates that
it will become the 3rd leading cause of death in less
than 20 years from now (Mathers and Loncar 2006;
Murray and Lopez 1997; WHO 2012). COPD is
characterised by airflow limitation that is not fully
reversible. It is usually progressive and associated
with an abnormal inflammatory response of the lung
to noxious particles or gases, most often from
cigarette smoke (Decramer et al. 2012). Up to
almost one quarter of the adults aged 40 years and
older may have mild airflow obstruction, according
to the latest prevalence surveys (Mannino and Buist
2007). One of the challenges in such a disease is to
identify patients at risk for brisk deterioration and to
develop diagnostic tools which are directly clinically
important (Agusti et al. 2010; Miravitlles et al.
2013).
Indications of COPD are production of sputum,
signs of dyspnea, chronic cough or/and a history of
exposure to the tobacco smoke (Rabe et al. 2007).
However, the diagnosis itself is based on measuring
differences in lung volume using a spirometer, as
most common signs of COPD and patient history
cannot accurately reflect COPD presence. Current
diagnosis is simple and inexpensive to perform, but
also lately debatable due to ability to overdiagnose
or underdiagnose (Garcia-Rio et al. 2011). Various
approaches have been developed to diagnose and
characterize COPD, either by measuring volatile
organic compounds in the exhaled air (Fens et al.
2009; Phillips et al. 2012), or by looking into
5
Marko T., Exadaktylos V., Aerts J., Troosters T., Decramer M., Berckmans D. and Janssens W..
Data based Modelling of Expired Airflow Clarifies Chronic Obstructive Pulmonary Disease.
DOI: 10.5220/0004735000050012
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2014), pages 5-12
ISBN: 978-989-758-011-6
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

computed tomography images (Bodduluri et al.
2013; Sorensen et al. 2012), or even by applying
forced oscillation technique to stimulate respiratory
system (Amaral et al. 2012). However, none of the
techniques entered clinical practice, due to their
complexity, costly undertaking or unsatisfactory
results.
Surprisingly, mathematical data-based modelling
was never performed when it comes to revealing
background of COPD. Starting from that point and
knowing that COPD, by its definition, is flow
limited (Decramer, Janssens, & Miravitlles 2012;
Dellaca et al. 2004) we hypothesized that modelling
of the flow dynamics during exhalation may offer a
more precise indication of COPD presence. This
should lead to better understanding of the COPD and
additional tool for diagnosis.
In the present study our objective was firstly to
develop a mathematical data-based model for the
decline of the forced expiratory flow. Secondly, to
investigate how the parameters from the model are
linked with COPD presence and its severity.
2 METHODS
2.1 Study Population
This study included data of 474 individuals who had
performed complete pulmonary function testing
(PFT) at cohort entry, including post-bronchodilator
spirometry, body plethysmography and diffusing
capacity. All included subjects were tested between
October 2007 and January 2009 at the University
Hospital of Leuven (Belgium), as described earlier
(Lambrechts et al. 2010; Wauters et al. 2011).
Briefly, participants were all current or former heavy
smokers with at least 15 pack-years and with
minimal age of 50 years. As COPD is smoking
disease per se, restricting our study to only smoking
individuals increased chances to observe more
abnormal pulmonary functions and patients with
higher risk for COPD. Individuals with suspicion or
diagnosis of asthma were excluded, as well as
patients with exacerbations due to COPD within last
6 weeks and patients with other respiratory diseases.
The study was approved by the local ethical
committee of the University Hospital Leuven, (KU
Leuven, Belgium). All patients included in the study
provided informed consent. The study design of the
LEUVEN COPD cohort can be found on
www.clinicaltrials.gov (NCT00858520).
According to the international COPD GOLD
guidelines (Rabe et al. 2007), patients with COPD
were identified when the post-bronchodilator
FEV1/FVC ratio was <0.7, furthermore they were
lined over different severity stages. The population
consisted of 336 patients with diagnosed COPD
comparing to 138 healthy controls. Stratified for
disease severity from mild (GOLD I) to moderate
(GOLD II), severe (GOLD III) and very severe
(GOLD IV), the COPD population was comprised of
77, 101, 97 and 61 patients respectively. Table 1
describes the population characteristics within two
separate groups, revealing typical characteristics for
smoking and demographics of COPD patients
admitted in hospitals.
2.2 Pulmonary Function Tests
All pulmonary function tests were performed with
standardized equipment (Masterlab, Erich Jeager,
Würzburg, Germany) by experienced respiratory
technicians, according to the ATS/ERS guidelines
(Miller et al. 2005). Spirometry data are post-
bronchodilator measures and expressed as percent
predicted of normal reference values (Quanjer et al.
1994).
Table 1: Study population characteristics; Values are
median and IQR; BMI = body mass index; M = male; F =
female; FEV1= forced expiratory volume in one second;
FVC = forced vital capacity; M = male; %pred. = percent
predicted of normal reference values.
Healthy COPD
Patients, N 138 336
Sex, M/F 110/28 260/76
Age, years 60.7(57.3– 64.6) 65.1(59.5– 72.1)
Smoking,
pack yr.
38.0(29.3– 52.0) 45.0(32.6– 60.0)
BMI, kg/m
2
26.4(24.0– 28.7) 25.0(22– 28)
FEV
1
, %pred. 104.0(94–112) 53.0(35– 78)
FVC, %pred. 108.0(100–118) 89.0(71– 106)
FEV
1
/FVC 0.75(0.73– 0.78) 0.47(0.37– 0.62)
2.3 Data based Modelling
To develop our data-based model we used
MATLAB (7.14, The MathWorks, Natick,
Massachusetts) and compatible toolbox for non-
stationary time series analysis, system identification,
signal processing and forecasting – CAPTAIN
toolbox (Taylor et al. 2007). In all individuals the
best expiratory curve (rule of highest sum of FEV1
and FVC (Miller et al. 2005)) within one spirometry
was exported from the Masterlab system at a
sampling rate of 125Hz. By extracting data points it
was possible to reconstruct the best expiratory
BIOSIGNALS2014-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
6
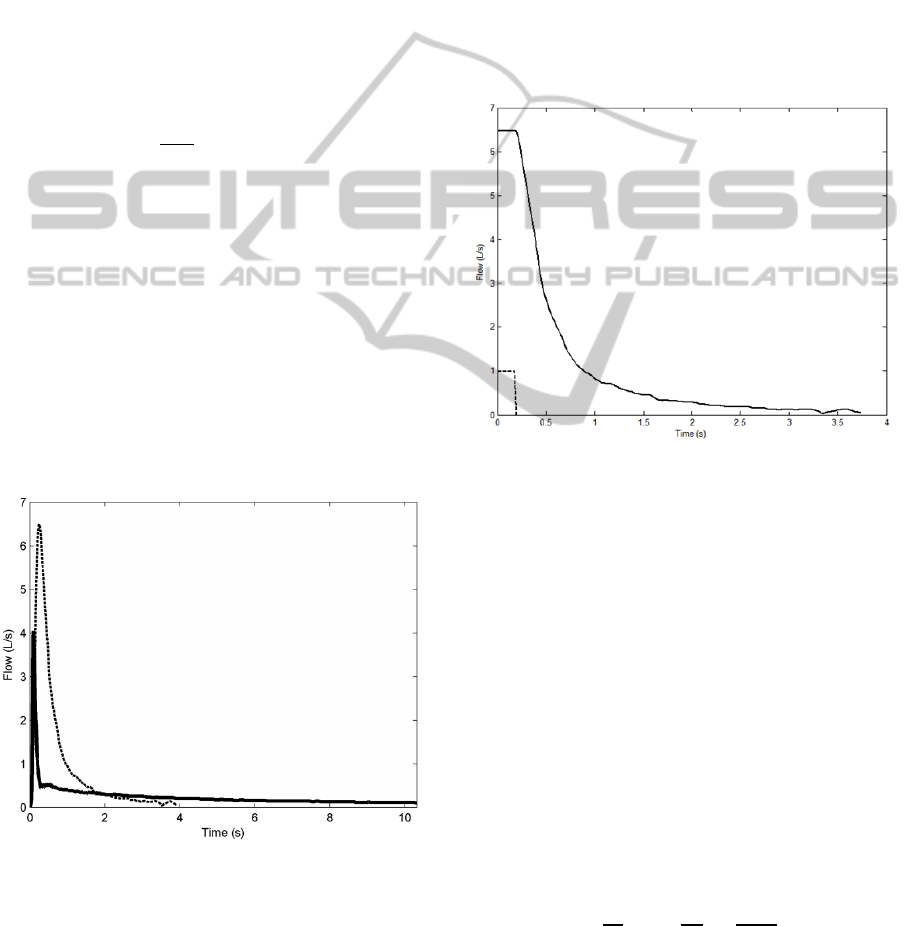
manoeuvre in MATLAB. To observe the dynamics
of the expiration, only the declining phase of
expiration was analysed. Declining is the area that
starts at peak flow and ends at the end of the
expiration, far right tail (Figure 1).
When starting with data-based modelling, the
appropriate model structure is determined using
objective methods of time series analysis from a
generic model class. The goal is to describe the data
in a parametrically efficient way, but still having
simplicity in the sense of model parameters and
model order. Considering our study and our data,
most appropriate model was a discrete-time transfer
function (TF) model for a single input single output
(SISO) system. The general form of such system is:
y
u
ξ
,
(1)
where y
is the output; u
is the input; ξ
is additive
noise, assumed to be zero mean; L is the backward
shift operator; A(L) and B(L) are polynomials
defined by the order of the model in the following
form:
A
L
1a
L⋯a
L
(2)
B
L
b
b
L⋯b
L
(3)
where n represents the order of the system: a
, …,
a
and b
, b
, …, b
are the TF denominator and
numerator parameters, respectively.
Figure 1: Two examples of expiratory manoeuvres; Solid
line represents expiratory flow of an individual with
diagnosed very severe COPD, while dashed line represents
expiratory flow of a healthy individual. Decline is
considered the section when the flow starts dropping from
its maximum back to its minimum, over time.
Once the input-output data are available, TF
parameters (Eq. (2) and (3)) can be identified using
statistical procedures. For the input data, we used
step-down for each model, while output signal was
original measurements obtained from spirometry.
The parameters of a TF model can be estimated
using various methods of identification and
estimation procedures (Ljung L. 1987; Young PC.
1984). In this study the Simplified Refined
Instrumental Variable (SRIV) algorithm was used as
a method for model identification. The advantage of
SRIV lays not only in yielding consistent estimates
of the parameters, but also in exhibiting close to
optimum performance in the model order reduction
context (Figure 2).
Figure 2: Step down (dashed line) used for each model as
input signal; Solid line represents an example of
declination, meaning output signal (different for each
individual). We assumed that the time-series of the output
had a constant value (first 10 data samples) then the drop
started, it was also point for the step-down of the input.
Based on these two signals SRIV estimates TF parameters.
An equally important problem to the parameter
estimation is the identification of the objective
model order which will result in low complexity.
The process of model order identification can be
performed by the use of well-chosen mathematical
measures which indicate the presence of over
parameterization. Often used successful
identification procedure to select the most
appropriate model structure is based on the
minimisation of the Young identification criterion,
(YIC) (Young 1981) (Eq. (4)).
YICln
σ
σ
ln
1
np
σ
p
a
(4)
where σ
is the sample variance of the model
residuals; σ
is the sample variance of the measured
DatabasedModellingofExpiredAirflowClarifiesChronicObstructivePulmonaryDisease
7
system output about its mean value; np is the total
number of model parameters; a
is the square of the
i-th element in the parameter vector a; p
is the i-th
diagonal element of the inverse cross product matrix
P(N); σ
p
can be considered as an approximate
estimate of the variance of the estimated uncertainty
on the i-th parameter estimate.
YIC is a heuristic statistical criterion which
consists of two terms, as shown in Eq. (4). The first
term provides a normalised measure of how well the
model fits the original data: the smaller the variance
of the model residuals, in relation to the variance of
the measured output, the smaller this term becomes.
The second term is a normalised measure of how
well the model parameter estimates are defined. This
term tends to become bigger when the model is
over-parameterised and the parameter estimates are
poorly defined. Consequently, the best model should
minimise the YIC and provide a good compromise
between goodness of fit and parametric efficiency.
Finally, upon passing all listed steps, derivation
of additional parameters which describe exhaled
airflow was feasible. Firstly, using an individual TF
for each subject, we were able to derive poles of the
model. These poles were direct representatives of
the dynamics of the observed model. Secondly, the
steady-state gain (SSG) of the model is also derived.
SSG is the ratio of the output and the input of the
model in steady state, and it is obtained by:
SSG
∆
y
∆u
∑
b
1
∑
a
(5)
3 RESULTS
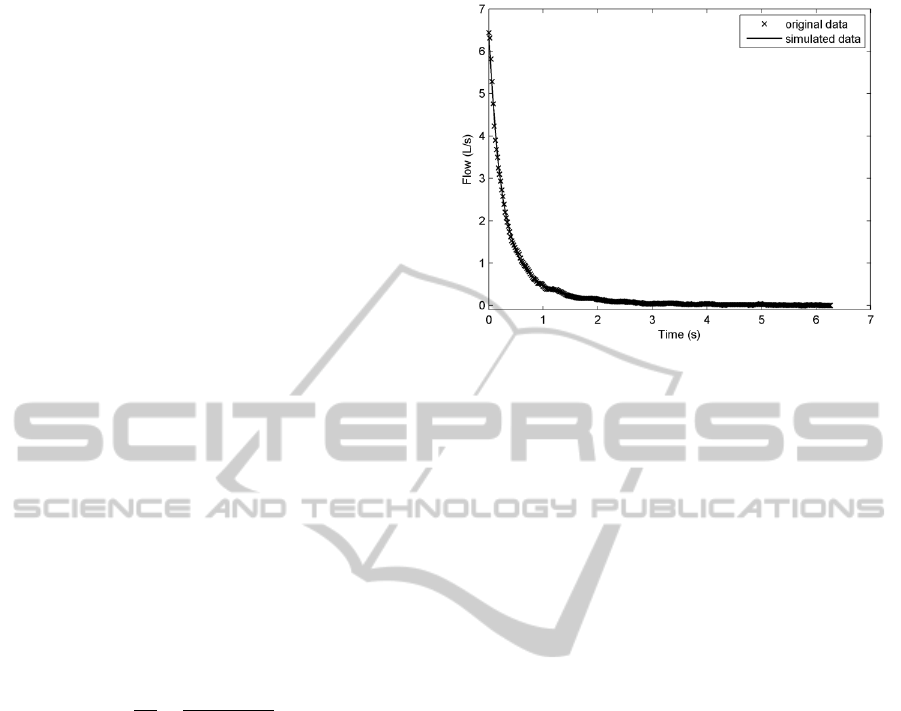
Using the already explained YIC, we discovered that
the most appropriate model would be a second-order
model. Looking into complete dataset, second-order
model explains data with a YIC of -14.5 (-15.7 – -
13.1) and R
T
2
of 0.997 (0.994 – 0.998) (values are
median and IQR). Confirmation of the good model
order identification is presented in Figure 3, where
the original output signal with the simulated one is
compared using the estimated parameters from
second-order model.
In total, analysis was performed employing two
poles (coming from second-order model) and SSG
of the model from 423 individuals. From the
included 474 individuals, 51 (=10.8%) had to be
excluded, where 32 (=6.8%) due to missing data
from the PFT and 19 (=4%) due to model instability.
More detailed investigation of poles of
the model, meaning the dynamics of the airflow
Figure 3: No difference between the original (marked with
x) and the simulated (solid line) output signal is noticed
(R
T
2
= 0.999, YIC = -17.6091) when using second-order
model.
exhalation, resulted in clear difference when
comparing subjects with and without COPD (see
figure 4). Certainly, first pole was higher when
COPD was present, indicating that the system starts
faster when disease occurs. Median (IQR) poles in
subjects without disease were 0.9868 (0.9810-
0.9892) and 0.9333 (0.9010-0.9529), respectively,
compared to 0.9929 (0.9901-0.9952) and 0.9082
(0.8669-0.9398) in subjects with COPD (p<0.0001
for first pole and p<0.001 for second pole).
Stratifying for disease severity, same shift in poles
with disease progression was noticed (Figure 5).
This pointed that the dynamics of the system
become faster with higher severity. Median poles
were 0.9895 and 0.9346 for GOLD 1, 0.9916 and
0.9160 for GOLD 2, 0.9946 and 0.9009 for GOLD 3
and finally for GOLD 4 0.9959 and 0.8615.
When focusing the analysis on the SSG of the
model, similar conclusions as the ones with poles
can be made. Median (IQR) SSG in subjects with
COPD was significantly lower 3.9 (2.7-5.6)
compared to 8.2 (7.1-9.3) in subjects without COPD,
(p<0.0001). When disintegrating over severity of
COPD, SSG decreased significantly (p<0.0001) with
each GOLD stage: 6.8 (5.7-7.8), 5.0 (3.9-5.7), 3.1
(2.6-3.7) and 2.3 (1.7-2.8), respectively (Figure 6).
This is manifested due to lower flow change that
occurs when lungs are obstructive compared to
healthy lungs.
BIOSIGNALS2014-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
8
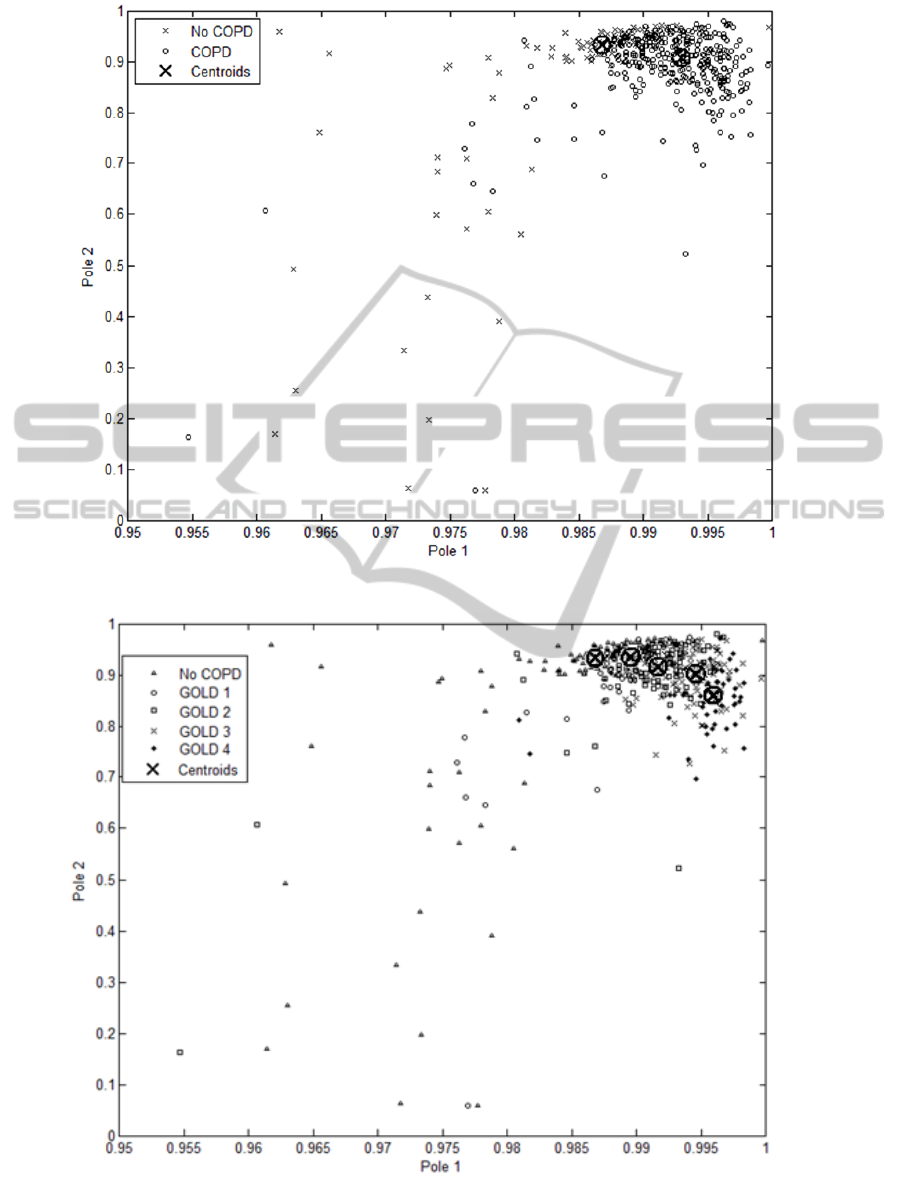
Figure 4: Pole distribution within two observed groups, with centroids pointing the median value of each group.
Figure 5: Distribution of poles when stratified for COPD severity (GOLD stages). Dynamics are faster with increase of
severity. Centroids indicate median values of each GOLD stage. We see movement of centroids from left to right, in the
order: from no COPD over each growing GOLD stage.
DatabasedModellingofExpiredAirflowClarifiesChronicObstructivePulmonaryDisease
9

Figure 6: Decrease of SSG with each GOLD stage.
4 CONCLUSIONS
Our study demonstrates that chronic obstructive
pulmonary disease observed at forced expiration can
be described by a second order data based model,
whereas model parameters relate very well with the
presence and severity of COPD. Our method
confirms that COPD is indeed a flow limited
disease, and in certain way raise a question whether
future diagnostic for COPD should go back to its
basis, its definition, and take flow values at
examination.
To the best of our knowledge, our study is the
first to validate the concept of COPD-associated
airflow dynamics in larger group of individuals
comprising COPD patients of all severity stages, as
well as smoking controls. In our population, we
found that poles and steady state gain match well
with severity of COPD. Interestingly, the estimated
model resulted in significantly lower steady state
gain within each severity stage. Moreover steady
state gain was significantly different comparing to
the healthy cases. This undoubtedly confirms that
obstructive lungs are having much more difficulties
to exhale flow and therefore, exhale it in much lower
speed.
The concept we are introducing opens new
opportunities for research in the field of respiratory
mechanism and respiratory diseases. In general, by
using airflow dynamics, with this study we provided
additional explanation of COPD behaviour. We see
that they anticipate that the faster dynamics of the
system are probability to notice presence of COPD
will increase. Moreover, with increase of dynamics,
the severity stages of COPD are also increasing.
This probably means that bigger obstruction of lungs
cause decreased exhalation of air which results in
faster emptying of the lungs (faster dynamics of the
exhalation). Various reasons influence such
occurrence, firstly it is common to observe airway
narrowing or airway collapse to cause suddenly
diminished airflow (Healy et al. 1984). Furthermore,
in COPD, the greatest reduction in air flow occurs
during expiration, as the pressure in the chest tends
to compress rather than expand the airways
(Koulouris and Hardavella 2011). One would
assume that loss of lung tissue elasticity, typical for
emphysematous type of COPD, plays additional role
in accelerating exhalation dynamics, as it might be
the case that lungs get faster its limits while exhaling
(Papandrinopoulou et al. 2012).
When comparing with the other alternative
approaches, advantage is that parameters obtained
from model-based method can have physiological
validity. Further, when used with routine spirometry
during patient examination, this method is de facto
simplest, fastest and cheapest to perform.
Additional strength of this study is the fact that
observing dynamics of the flow decay represents
same approach that many researchers had performed
in the past, but based only on a visual basis of
typical patterns (Bass 1973; Jayamanne et al. 1980).
Today routinely, clinicians are capable to presume
presence of Chronic Obstructive Pulmonary Disease,
on the basis of visual assessment of flow decay,
whereas with this study we offer more precise and
automated way of inspection. Furthermore, we
believe that the concept which we are introducing, is
easy to understand and linked to physiological
behaviour of the lungs. Moreover, we believe that
extra value of this study comes from the study
cohort itself. All patients are heavy smokers older
than 50 years, meaning that they are all labelled as
having risk of COPD, consequently inducing bigger
challenge to distinguish between diseased and not
diseased.
Finally, our method failed to provide valid
measurements in 4% of the cases. This occurrence is
inevitable, as we tried to automatize process where
data selection and estimation algorithm are not
always the optimal ones. Certainly, this could be
avoided in most of the cases, if ensuring that
exhalation ends with plateau (having stable ending).
Taken together, our data provide strong evidence
that dynamics of the forced exhaled air can be used
to get elevated understanding of the chronic
obstructive pulmonary disease. Moreover, if
characterized like in our model, flow decline can be
used to access Chronic Obstructive Pulmonary
Disease by spirometry.
BIOSIGNALS2014-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
10

ACKNOWLEDGEMENTS
The authors would like to thank Geert Celis and co-
workers (Respiratory Division, University Hospital
Leuven, Belgium) for helping in collection of patient
data and their technical support in extracting data
from the Masterlab.
REFERENCES
Agusti, A., Calverley, P. M., Celli, B., Coxson, H. O.,
Edwards, L. D., Lomas, D. A., MacNee, W., Miller, B.
E., Rennard, S., Silverman, E. K., Tal-Singer, R.,
Wouters, E., Yates, J. C., & Vestbo, J. 2010.
Characterisation of COPD heterogeneity in the
ECLIPSE cohort. Respir.Res., 11, 122 available from:
PM:20831787.
Amaral, J. L., Lopes, A. J., Jansen, J. M., Faria, A. C., &
Melo, P. L. 2012. Machine learning algorithms and
forced oscillation measurements applied to the
automatic identification of chronic obstructive
pulmonary disease. Comput.Methods Programs
Biomed., 105, (3) 183-193 available from:
PM:22018532.
Bass, H. 1973. The flow volume loop: normal standards
and abnormalities in chronic obstructive pulmonary
disease. Chest, 63, (2) 171-176 available from:
PM:4688062.
Bodduluri, S., Newell, J. D., Jr., Hoffman, E. A., &
Reinhardt, J. M. 2013. Registration-based lung
mechanical analysis of chronic obstructive pulmonary
disease (COPD) using a supervised machine learning
framework. Acad.Radiol., 20, (5) 527-536 available
from: PM:23570934.
Decramer, M., Janssens, W., & Miravitlles, M. 2012.
Chronic obstructive pulmonary disease. Lancet, 379,
(9823) 1341-1351 available from: PM:22314182.
Dellaca, R. L., Santus, P., Aliverti, A., Stevenson, N.,
Centanni, S., Macklem, P.T., Pedotti, A., & Calverley,
P.M. 2004. Detection of expiratory flow limitation in
COPD using the forced oscillation technique.
Eur.Respir.J., 23, (2) 232-240 available from:
PM:14979497.
Fens, N., Zwinderman, A. H., van der Schee, M. P., de
Nijs, S. B., Dijkers, E., Roldaan, A. C., Cheung, D.,
Bel, E. H., & Sterk, P. J. 2009. Exhaled breath
profiling enables discrimination of chronic obstructive
pulmonary disease and asthma. Am.J.Respir.Crit Care
Med., 180, (11) 1076-1082 available from:
PM:19713445.
Garcia-Rio, F., Soriano, J. B., Miravitlles, M., Munoz, L.,
Duran-Tauleria, E., Sanchez, G., Sobradillo, V., &
Ancochea, J. 2011. Overdiagnosing subjects with
COPD using the 0.7 fixed ratio: correlation with a poor
health-related quality of life. Chest, 139, (5) 1072-
1080 available from: PM:21183609.
Healy, F., Wilson, A. F., & Fairshter, R. D. 1984.
Physiologic correlates of airway collapse in chronic
airflow obstruction. Chest, 85, (4) 476-481 available
from: PM:6705575.
Jayamanne, D. S., Epstein, H., & Goldring, R. M. 1980.
Flow-volume curve contour in COPD: correlation with
pulmonary mechanics. Chest, 77, (6) 749-757 available
from: PM:7398386.
Koulouris, N. G. & Hardavella, G. 2011. Physiological
techniques for detecting expiratory flow limitation
during tidal breathing. Eur.Respir.Rev., 20, (121) 147-
155 available from: PM:21881143.
Lambrechts, D., Buysschaert, I., Zanen, P., Coolen, J.,
Lays, N., Cuppens, H., Groen, H. J., Dewever, W., van
Klaveren, R. J., Verschakelen, J., Wijmenga, C.,
Postma, D. S., Decramer, M., & Janssens, W. 2010.
The 15q24/25 susceptibility variant for lung cancer and
chronic obstructive pulmonary disease is associated
with emphysema. Am.J.Respir.Crit Care Med., 181,
(5) 486-493 available from: PM:20007924.
Ljung L. 1987. System Identification: Theory for the User
Englewood Cliffs, NJ: Prentice-Hall.
Mannino, D. M. & Buist, A.S. 2007. Global burden of
COPD: risk factors, prevalence, and future trends.
Lancet, 370, (9589) 765-773 available from:
PM:17765526.
Mathers, C. D. & Loncar, D. 2006. Projections of global
mortality and burden of disease from 2002 to 2030.
PLoS.Med., 3, (11) e442 available from:
PM:17132052.
Miller, M. R., Hankinson, J., Brusasco, V., Burgos, F.,
Casaburi, R., Coates, A., Crapo, R., Enright, P., van
der Grinten, C. P., Gustafsson, P., Jensen, R., Johnson,
D. C., MacIntyre, N., McKay, R., Navajas, D.,
Pedersen, O. F., Pellegrino, R., Viegi, G., & Wanger, J.
2005. Standardisation of spirometry. Eur.Respir.J., 26,
(2) 319-338 available from: PM:16055882.
Miravitlles, M., Soler-Cataluna, J. J., Calle, M., &
Soriano, J. B. 2013. Treatment of COPD by clinical
phenotypes: putting old evidence into clinical practice.
Eur.Respir.J., 41, (6) 1252-1256 available from:
PM:23060631.
Murray, C. J. & Lopez, A. D. 1997. Alternative
projections of mortality and disability by cause 1990-
2020: Global Burden of Disease Study. Lancet, 349,
(9064) 1498-1504 available from: PM:9167458.
Papandrinopoulou, D., Tzouda, V., & Tsoukalas, G. 2012.
Lung compliance and chronic obstructive pulmonary
disease. Pulm.Med., 2012, 542769 available from:
PM:23150821.
Phillips, C. O., Syed, Y., Parthalain, N. M., Zwiggelaar,
R., Claypole, T. C., & Lewis, K. E. 2012. Machine
learning methods on exhaled volatile organic
compounds for distinguishing COPD patients from
healthy controls. J.Breath.Res., 6, (3) 036003 available
from: PM:22759349.
Quanjer, P. H., Tammeling, G. J., Cotes, J. E., Pedersen,
O. F., Peslin, R., & Yernault, J. C. 1994. [Lung
volumes and forced ventilatory flows. Work Group on
Standardization of Respiratory Function Tests.
European Community for Coal and Steel. Official
DatabasedModellingofExpiredAirflowClarifiesChronicObstructivePulmonaryDisease
11

position of the European Respiratory Society].
Rev.Mal Respir., 11 Suppl 3, 5-40 available from:
PM:7973051.
Rabe, K. F., Hurd, S., Anzueto, A., Barnes, P. J., Buist, S.
A., Calverley, P., Fukuchi, Y., Jenkins, C., Rodriguez-
Roisin, R., van, W. C., & Zielinski, J. 2007. Global
strategy for the diagnosis, management, and prevention
of chronic obstructive pulmonary disease: GOLD
executive summary. Am.J.Respir.Crit Care Med., 176,
(6) 532-555 available from: PM:17507545.
Sorensen, L., Nielsen, M., Lo, P., Ashraf, H., Pedersen, J.
H., & de, B. M. 2012. Texture-based analysis of
COPD: a data-driven approach. IEEE
Trans.Med.Imaging, 31, (1) 70-78 available from:
PM:21859615.
Taylor, C. J., Pedregal, D. J., Young, P. C., & Tych, W.
2007. Environmental time series analysis and
forecasting with the Captain toolbox. Environmental
Modelling & Software, 22, (6) 797-814.
Wauters, E., Smeets, D., Coolen, J., Verschakelen, J., De,
L. P., Decramer, M., Vansteenkiste, J., Janssens, W., &
Lambrechts, D. 2011. The TERT-CLPTM1L locus for
lung cancer predisposes to bronchial obstruction and
emphysema. Eur.Respir.J., 38, (4) 924-931 available
from: PM:21622582.
WHO. World health statistics 2008. http://www.who.int/
whosis/whostat/EN_WHS08_Full.pdf. Accessed 2012.
Young P. C. 1984. Recursive Estimation and Time-Series
Analysis Berlin: Springer.
Young, P. 1981. Parameter-Estimation for Continuous-
Time Models - A Survey. Automatica, 17, (1) 23-39
available from: ISI:A1981LE26600003.
BIOSIGNALS2014-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
12
