
Non-invasive Pain Sensor Development for Advanced Control Strategy of
Anesthesia
A Conceptual Study
Dana Copot, Am
´
elie Chevalier, Clara M. Ionescu and Robin De Keyser
Department of Electrical energy, Systems and Automation, Ghent University,
Sint-Pietersnieuwstraat 41, Blok B2, 9000, Gent, Belgium
Keywords:
Compartmental Fractional Derivative Models, Fractional-Order Impedance Models (FOIM), Model-based
Predictive Control (MPC), Non-invasive Pain Sensor.
Abstract:
This paper introduces the mechanisms of pain perception in the human body in order to start the challenging
task of controlling analgesia as part of general anesthesia. This research proposes a pain sensor, which mea-
sures analgesia levels. For control purpose, a prediction model is needed in order to obtain a model-based
predictive control (MPC) strategy. This paper proposes to employ a compartmental fractional-order derivative
model as a prediction model for the diffusion process that occurs when a drug is taken up by the human body.
Simulations are preformed to investigate the effect of the fractional order on the diffusion of Propofol in the
human body.
1 INTRODUCTION
The main objective of a drug delivery system is to pro-
vide effective therapy by minimizing the side effects
and reducing deviation from the desired state of the
patient. To develop an advanced drug delivery sys-
tem, there is a need for a mathematical model, which
takes pharmacodynamic and pharmacokinetic effects
of the drugs into account.
Nowadays, the applications of control theory rely
mostly on deterministic assumptions where the gen-
eral approach of open-loop configuration assumes
that the pharmacokinetic relations can be modeled
by a linear system with known parameters (Wagner,
1976). Nevertheless, these assumptions do not take
into account the individuality of each patient. Fur-
thermore, is the model optimization procedure based
on the average of a population which results in sub-
optimal solutions. Therefore, the challenge is to use
a closed-loop configuration in order to formalize the
process of observation and intervention to provide a
better and more accurate control.
Moreover, some computer-control systems try to
predict the future drug effect in order to adjust the
parameters in advance (Absalom et al., 2011). As
anesthesia is neither a simple process nor a well-
understood process, it is a very challenging system
to control. Taking into account the difficulty in mod-
eling consciousness, the mechanism of anesthetic-
induced loss of consciousness is nowadays still con-
founding scientists. Current models for anesthe-
sia are mean field models of drug action (Absalom
et al., 2011), which describe anesthetic phenomena
based on the electroencephalogram (EEG) and asso-
ciated with different brain states. A relationship be-
tween changes in EEG signals and depth of anes-
thesia was developed in order to control the deliv-
ery of intravenous drugs. Afterwards, this relation-
ship was used to inject liquid ether into an anes-
thetic circuit. The first main impulse was given in the
early 70s with the introduction of physiology-based
compartment-models of uptake and action of anes-
thetic drugs (Zwart et al., 1972).
The advantage of automated closed loop control
of anesthesia is a continuous drug delivery contrary
to intermittent control, which is nowadays standard
practice. By having a continuous drug delivery, over-
dose or under-dose of hypnotic or analgesic drugs
can be avoided. Under-dosing patients can result in
sensing pain during the surgery without being able
to move. However, feedback information currently
presents a major problem for control algorithms be-
cause of the presence of artifacts (e.g. eye movement,
leg movement, etc.) or by patient mismatch, which
results in erroneous signals. Consequently, the qual-
ity of measured signals decreases, leading to complex
95
Copot D., Chevalier A., M. Ionescu C. and De Keyser R..
Non-invasive Pain Sensor Development for Advanced Control Strategy of Anesthesia - A Conceptual Study.
DOI: 10.5220/0004742800950101
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2014), pages 95-101
ISBN: 978-989-758-013-0
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

numerical filtering.
The paper is structured as follows: in section II,
we describe how pain is perceived and transmitted
during anesthesia. The challenges coinciding the au-
tomated control of anesthesia are discussed in section
III, followed by the discussion of the proposed sensor.
Section IV shows the simulations and results. In the
last section conclusions are formulated.
2 PAIN PERCEPTION AND
TRANSMISSION DURING
ANESTHESIA
Anesthesia is the process of having sensation (includ-
ing the feeling of pain) blocked or temporarily taken
away. Adequate anesthesia can be defined as a re-
versible pharmacological state where the three main
parts of anesthesia (muscle relaxation, hypnosis and
analgesia) are guaranteed. Good monitoring of anes-
thesia includes an attentive observation of the patient
during critical phases. There are several kinds of gen-
eral anesthetics, but those most commonly used en-
hance or mimic the action of the inhibitory neuro-
transmitter gamma-aminobutyric acid (GABA) (Gar-
cia et al., 2010).
The main components of anesthesia are: (1) mus-
cle relaxation, which is induced to prevent unwanted
movement or muscle tone and causes paralysis dur-
ing surgical procedures. EMG signals are used to
quantify the level of muscle relaxation. (2) hypno-
sis, which is a general term indicating unconscious-
ness and absence of post operative recall of events oc-
curred during surgery (Kuizenga et al., 2001). Level
of hypnosis is related with the infusion of drugs and
can be monitored by a BIS monitor (based on EEG)
and (3) analgesia (pain relief) which is an insensibil-
ity to pain without loss of consciousness i.e. a state
in which painful stimuli are not perceived or not in-
terpreted as pain. It is usually induced by an anal-
gesic drug, although trauma or a disease may produce
a general or regional analgesia. Figure 1 shows the
input-output variables of anesthesia. Notice, that the
three main parts of anesthesia are unmeasurable up
until now. However, relationships have been defined
between unmeasurable and measurable outputs in or-
der quantify the levels of hypnosis and relaxation. For
analgesia levels ,however, these methods are insuffi-
cient.
Understanding pain perception and transmission
is necessary in order to measure analgesia during gen-
eral anesthesia. Pain receptors are distributed in the
superficial layers of the skin (Figure 2) or in some
HUMAN BODY
i.v. ANESTHETICS
VOLATILE ANESTHETICS
MUSCLE RELAXANTS
VENTILATION PARAMETERS
NaCl
SURGICAL STIMULUS
BLOOD LOSS
MANIPULATED
VARIABLES
DISTURBANCES
HYPNOSIS
ANALGESIA
RELAXATION
EEG PATTERN
HEART RATE
CO
2
CONC.
BLOOD PRESSURE
INSP/EXP CONC.
UNMEASUREABLE
OUTPUTS
MEASUREABLE
OUTPUTS
Figure 1: Schematic of input/output variables of anesthesia.
internal tissues. Five different types of receptors ex-
ist: (1) mechanoreceptors, which detect mechanical
deformation of the receptor or its adjacent cells; (2)
thermoreceptors, which detect changes in tempera-
ture; (3) nociceptors, which detect the damage of the
tissues, whether it be physical or chemical damage;
(4) electromagnetic receptors, which detect light on
the retina of the eye and (5) chemoreceptors, which
detect the taste in the mouth, smell in the nose, oxy-
gen levels in the arterial blood, carbon dioxide con-
centration and other factors that make up the chem-
istry of the body. Pain receptors are activated by ex-
tremes of pressure and temperature or as a veritable
soup of chemicals released from injured tissue. His-
tamine, K
+
, ATP (AdenasineTriPhosphate), acids and
bradykinin are among the most potent pain producing
chemicals (Keele, 1970).
Figure 2: Schematic overview of pain receptors in the skin
(Marieb and Hoehn, 2011).
Pain perception has three stages: (1) a peripheral
stage peripheral tissue sensitization, (2) a transmis-
sion stage - by specialized structures and (3) an inte-
gration of pain - can be conscious or not and involves
functions such as: attention, concentration, memory,
affect.
Peripheral stage - in this first stage tissues are
damaged due to chemical, mechanical or thermal
stimuli followed by stimulation of pain receptor and
activation of the receptors by noxious stimuli. When
an inflammation appears the pain fibers are subdued
to chemical aggression. Once the tissue is damaged,
the release of chemical substances (e.g. bardykinin)
takes place. This leads to a sensitization of nerve end-
ings that results in a pain signal and an increase in
local temperature (Marieb and Hoehn, 2011).
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
96
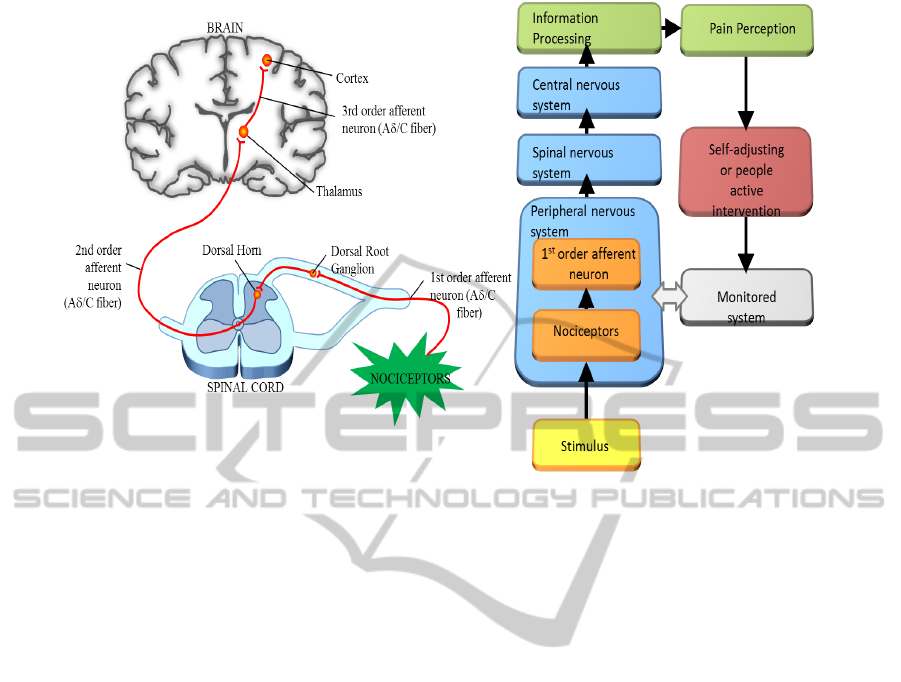
Figure 3: Schematic representation of a biological pain pathway (right side) and general flow chart of the pain perception
process (left side).
Transmission stage - the second stage includes:
peripheral receptors, nervous tracts, spinal mecha-
nisms, ascending and descending pathways, brain-
stem, thalamus and the cortex. According to control
theory of pain (Melzack, 2011), the mechanism in
the brain acts as a gate to increase/decrease the flow of
nerve signals from the peripheral receptors to the cen-
tral nervous system. Pain transmission is influenced
by many factors such as: (a) a continuous increase of
duration and amplitude of the action potentials in the
dorsal horn, this phenomenon is named wind-up phe-
nomenon, (b) a correlation of the amplification phe-
nomenon according to the stimulation of fibers. Clin-
ically, this results in hyperalgesia or secondary pain
and the phenomenon is described as a temporal sum-
mation and (c) a central hypersensitivation, this rep-
resents the pathophysiological mechanism of chronic
pain.
Integration stage - the last stage involves com-
ponents such as brainstem, thalamus, hypothalamus,
subcortical nuclei and brain cortex. These structures
take care of flexion reflexes, pain sensation and its
perception, responses, etc. Pain control depends on
the thalamus and the gate system.
A schematic representation of the pain pathway is
presented in figure 3. The incoming pain fibers excite
second-order neurons that send long fibers to the op-
posite side of the cord and then upward to the brain
as can be observed in figure 3 (right hand side). Fig-
ure 3 (left hand side) shows a general flow chart that
describes the pain perception process.
3 CHALLENGES FOR
AUTOMATED CONTROL OF
ANESTHESIA
The development of a pain sensor to measure analge-
sia levels during general anesthesia has several chal-
lenges.
• The first step is to obtain a suitable patient model.
Taking into account the individuality of each pa-
tient the identification is very challenging.
• The second challenge of this research is that this
model has to be generalized.
To control the depth of the anesthesia, there is a
need for a sensor that can measure the level of analge-
sia. The degree to which a person reacts to pain varies
tremendously. A robust controller should handle the
nonlinear response profile and inter- and intra-patient
variation of the patient’s analgesic state to infusion
of an analgesic drug (e.g. Reminfetanil, Propofol).
An ideal controller guides the induction of anesthesia
in order to reach the target as fast as possible with-
out initial overshoot and maintains the desired tar-
get. Therefore, from control engineering viewpoint,
model-based predictive control (MPC) plays a crucial
role in solving such complex problems.
Non-invasivePainSensorDevelopmentforAdvancedControlStrategyof
Anesthesia-AConceptualStudy
97
One proposed benefit of automated, closed-loop
drug delivery systems is that continuous, responsive
control of the clinical and therapeutic effect may im-
prove quality of care compared to intermittent control.
3.1 Non-invasive Pain Sensor
Development
Pain is a complex response to the interaction of mul-
tiple inflammatory mediators that are released at the
point of injury. Clinical assessment of pain is neces-
sary to diagnose, manage and choose treatment op-
tions, as well as for the evaluation of the most effi-
cient treatment. Current methods of assessing pain
include visual analog, Wong-Baker faces and verbal
numeric scales (Hemmerling et al., 2007). However,
studies have shown that these methods may be sub-
jective and discrepancies are likely to be recorded for
similar pain intensity measurements. Hence, there is
a need to develop a more objective scale that relies on
the fundamentals of biochemical mechanisms of pain
transduction.
General anesthesia consists of three components
acting simultaneously on the patient’s vital signs:
hypnosis, analgesia and neuromuscular blockade.
Hypnosis and neuromuscular blockade are relatively
well-characterized and can be quantified by respec-
tively electroencephalogram (EEG) and electromyo-
gram (EMG) data. By contrast, analgesia is far from
being well-characterized and no sensor is available for
measuring the pain relief levels that the patient ex-
periences during general anesthesia. The challenge
originates from unavailability of models that charac-
terize pain perception in the neural dynamics. Un-
like the well-understood dose-response relationship
for the hypnotic component of sedation, the dose-
response relationship for the analgesic component of
sedation needs further study.
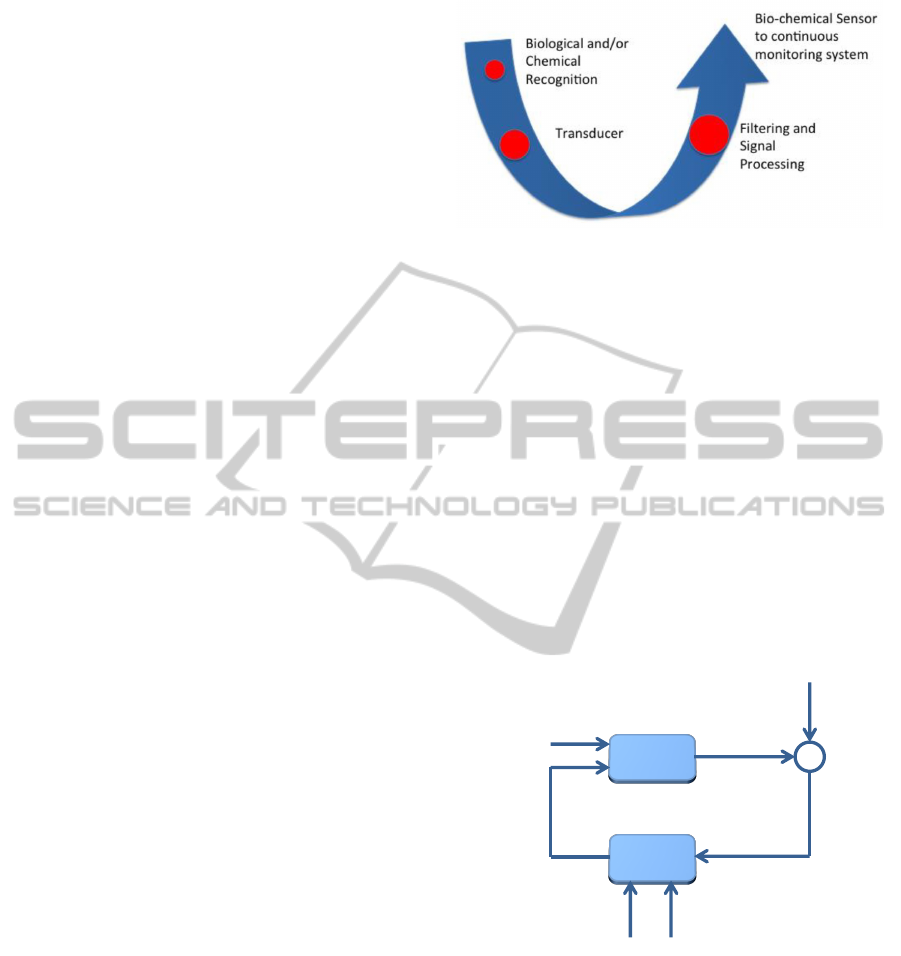
Figure 4 shows the three main parts proposed for
a pain sensor: the role of biological recognition ele-
ments (receptors, enzymes, antibodies, etc.) is to dif-
ferentiate the target molecules in the presence of var-
ious chemicals, the transducer electrochemical, opti-
cal, magnetic, etc.) converts the bio-recognition event
into a measurable signal, the signal processing part
converts the signal into a readable form.
3.2 A Prediction Model for
Model-based Predictive Control
Model-based Predictive Control (MPC) refers to a
family of control algorithms that compute a sequence
of manipulated variables by solving an optimiza-
tion problem, incorporating explicit knowledge of the
Figure 4: Three main parts of the proposed sensor.
plant model and incorporating feedback information
(De Keyser, 2003). Due to the properties of MPC
(its ability to handle nonlinear, constrained, and mul-
tivariable systems but also its severe computational
requirements), it has primarily been used in the chem-
ical process industries. Thus, while MPC remains an
open and growing area of research in systems and
control, there are somewhat limited applications re-
ported outside the processes industries. More re-
cently, there has been considerable interest in ex-
panding the applicability of MPC to other domains
of engineering which were traditionally considered
unsuitable for MPC due to their small physical size
and fast dynamics. The MPC strategy can be visual-
ized by the block-scheme in Figure 5 (De Keyser and
Van Cauwenberghe, 1981; D’hulster et al., 1983).
Model
Optimizer
Cost Function
Constraints
Future Errors
Future Inputs
Past Inputs
and Outputs
Predicted
Outputs
Reference
Trajectory
-
+
Figure 5: MPC block-scheme.
The success of MPC as a computer control
paradigm can be attributed to three important factors.
First and foremost is the incorporation of an explicit
process model into the control calculation; this allows
the controller to deal directly with all significant fea-
tures of the system dynamics. Second, the MPC al-
gorithm predicts the system behavior over a future
horizon in time. This means that the effects of distur-
bances can be anticipated and removed, allowing the
controller to drive the system more closely along the
desired trajectory. Finally, the MPC controller con-
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
98
siders input, state and output constraints directly in
the control calculation. This means that constraint vi-
olations are far less likely, resulting in tighter control
around the optimal operating point of the system.
Standard models include step response models,
transfer function models and linear state space mod-
els, however, these models do not suffice in model-
ing the dynamics of the diffusion processes that oc-
cur in the human body. To model the diffusion pro-
cesses in the human body, we use compartmental
models in combination with fractional-order deriva-
tives i.e. Fractional Calculus (Dokomuetzidis et al.,
2010). Two compartments are used in this diffusion
model: plasma (blood) and muscle.
3.2.1 Fractional Calculus Principles
Fractional Calculus is a generalization of integration
and derivation to non-integer (fractional) order oper-
ators (West, 1990). At first, we generalize the dif-
ferential and integral operators into one fundamental
operator D
n
t
(n is the operation order) which is known
as fractional calculus. Several definitions of this op-
erator have been proposed. All of them generalize
the standard differentialintegral operator in two main
groups: (a) they become the standard differential inte-
gral operator of any order when n is an integer; (b) the
Laplace transform of the operator D
n
t
is s
n
(provided
zero initial conditions), and hence the frequency char-
acteristic of this operator is ( jω)
n
. The latter is very
appealing for the design of parametric modeling and
control algorithms by using specifications in the fre-
quency domain. A fundamental D
n
t
operator, a gener-
alization of integral and differential operators (differ-
integration operator), as shown in equation (1).
D
n
t
=
d
n
dt
n
, n > 0
1, n = 0
R
t
0
(dα)
−n
, n < 0
(1)
In this equation n is the fractional order (FO) and
dα is the derivative function. Since the entire re-
search will focus on the frequency domain approach
for fractional order derivatives and integrals, we shall
not introduce the complex mathematics for time do-
main analysis. The Laplace transform for integral and
derivative order n are, respectively:
L{D
−n
t
f (t)} = s
−n
F(s) (2)
L{D
n
t
f (t)} = s
n
F(s) (3)
where F(s) = L{ f (t)} and s is the Laplace complex
variable. The Fourier transform can be obtained by
replacing s by jω in the Laplace transform and the
equivalent frequency-domain expressions are:
1
( jω)
n
=
1
ω
n
cos
nπ
2
− jsin
nπ
2
(4)
( jω)
n
= ω
n
cos
nπ
2
+ jsin
nπ
2
(5)
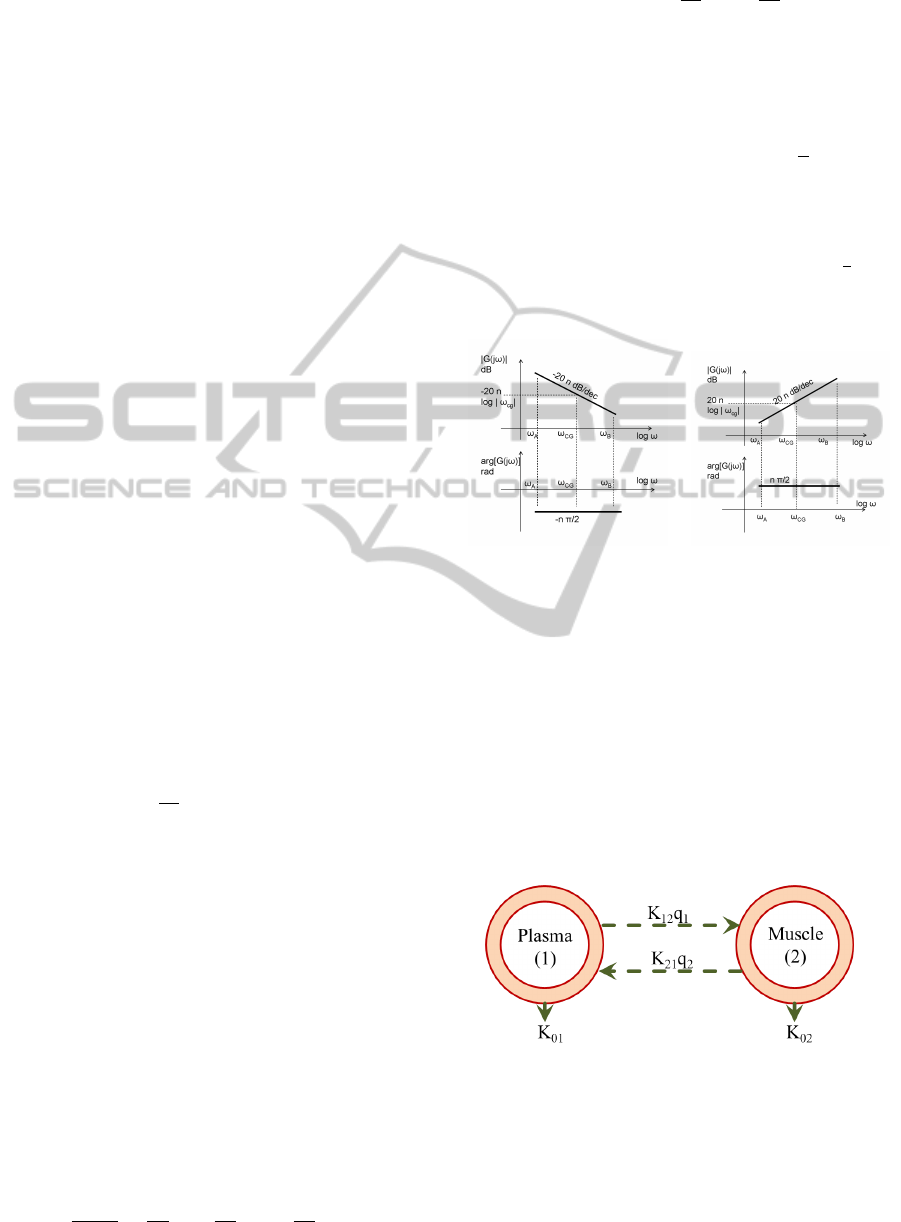
Thus, the modulus and the argument of the FO terms
are given by:
Modulus(dB) = 20 log
( jω)
∓n
= ∓20nlog
|
ω
|
(6)
Phase(rad) = arg
( jω)
∓n
= ∓n
π
2
(7)
resulting in a straight line with a slope of ∓20n pass-
ing through 0 dB for ω = 1 for the magnitude (dB
vs. log-frequency), respectively a horizontal line,
thus independent with frequency, with value ∓n
π
2
for
the phase (rad vs. log-frequency). The respective
sketches are given in figure 6.
Figure 6: Sketch representation of the FO integral and
derivator operators in frequency domain, by means of the
Bode plots (magnitude above and phase below).
3.2.2 Principles of Compartmental Fractional
Derivative Models
A two-compartmental fractional derivative model can
be used to model the diffusion process in the human
body (Popovic et al., 2010; Beneken and van Oost-
rom, 1998). The model is formulated so that the mass
balance is preserved. In figure 7, we see a conceptual
schematic of a model.
Figure 7: Conceptual schematic of a two compartment
model.
The first compartment represents the plasma or
any other region in the body where the kinetics of
the drug are uniform. The second compartment repre-
sents the place where the drug is applied i.e. muscle,
subcutaneous tissue or digestive tract. Traditionally,
Non-invasivePainSensorDevelopmentforAdvancedControlStrategyof
Anesthesia-AConceptualStudy
99
the two compartments are described by a system of
differential equations of integer order.
˙q
1
(t) = −K
12
q
1
(t) (8)
˙q
2
(t) = K
12
q
1
(t) − K
02
q
2
(t) (9)
Recently, the fractional-order models seem to better
suit the dynamics of biological systems than the in-
teger one (Magin, 2010). A simple model of a two-
compartmental system is then given by the following
equations:
τ
α
1
−1
1
0
D
α
1
t
q
1
(t) = −K
12
q
1
(t), (10)
τ
α
2
−1
2
0
D
α
2
t
q
2
(t) = K
12
q
1
(t) − K
02
q
2
(t), (11)
where we assumed K
01
= 0, K
21
= 0 and with the ini-
tial conditions q
1
(0) = dose, and q
2
(0) = 0. In these
equations τ
1
and τ
2
are time constants which repre-
sent the speed of diffusion, while n
1
and n
2
represent
a non-integer between 0 and 1 and characterize the
type of diffusion (sub-, super-, etc).
4 SIMULATIONS AND RESULTS
In this section we presents a first attempt in using
the fractional order derivatives in modelling Propo-
fol concentration. Based on the fractional-order two-
compartmental model mentioned in the previous sec-
tion, simulations are performed. In our simulations,
the anesthetic drug is Propofol. The objective of
this simulations is to investigate the influence of the
fractional-order derivatives on the Propofol concen-
tration in a target organ i.e. the muscle.
We simulate the diffusion of Propofol in a patient
of 45 years old that weighs 90 kg and has a height of
184 m. We take a bolus injection of 3.33 mg/s during
a period of 15 seconds, which corresponds with an
injected amount of drug of 50 mg.
The values of K
12
and K
02
are dependent on the
age of the patient and are calculated as follows:
K
02
= 1.29 − 0.024 ∗ (age − 53) (12)
K
12
=
K
02
v
1
(13)
with v
1
the volume of blood in liters (v
1
= 4,27`).
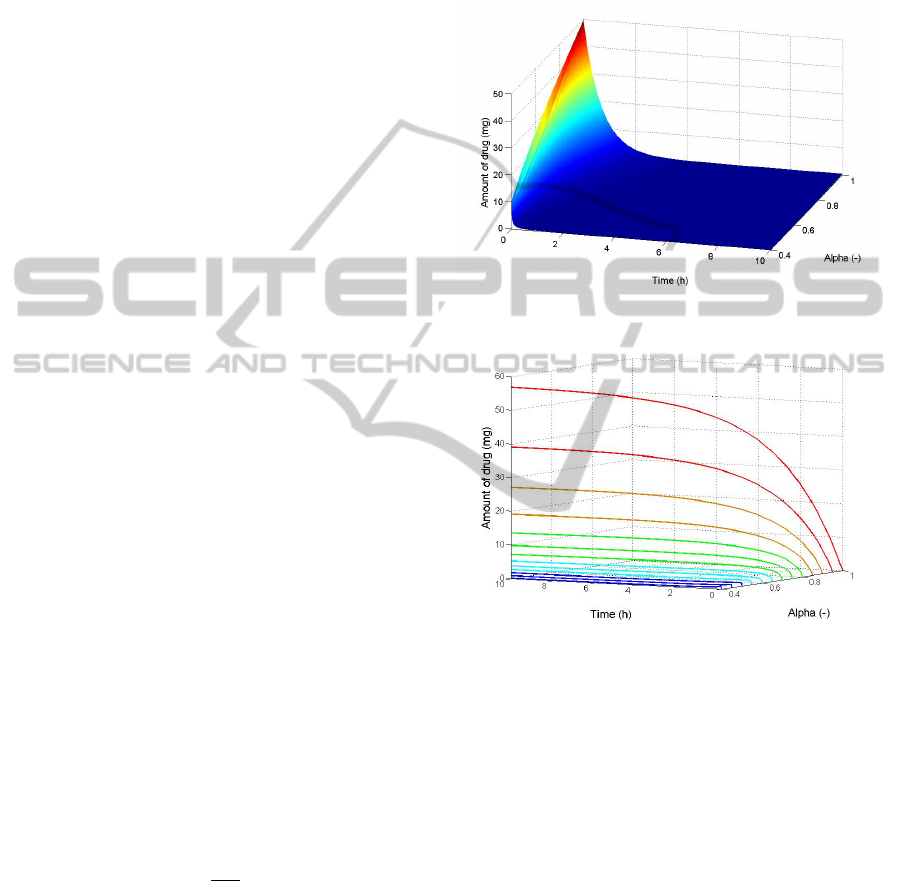
Figure 8 shows the amount of Propofol in com-
partment 1, i.e. the bloodstream, as a function of time
and the fractional order α. In figure 9 we see the
amount of drug for compartment 2, i.e. the muscle,
in function of time at distinct values of α. From fig-
ure 8 we can conclude that the decay of the numerical
solution changes in function of α. For lower values
of α, we observe higher decay rates, which show that
fractional-order models can capture inter-patient vari-
ability. In figure 9 we can see that the higher values
of α will result in higher amounts of drug taken up
by the muscle. This can be explained by the slower
decay rates in the bloodstream resulting in more time
for the drug to diffuse through the membrane.
Figure 8: Amount of drug in compartment 1 as a function
of α and time.
Figure 9: Amount of drug in compartment 2 as a function
of α and time.
5 CONCLUSIONS
Controlling analgesia levels during general anesthe-
sia is of great importance for the patient’s quality of
treatment. This research gives a overview of the com-
plexity of the neurophysiological pain perception pro-
cess needed to understand analgesia. A non-invasive
pain sensor has to be developed to ensure feedback in
the analgesia control strategy. The main challenges in
this development is the patient model. A model-based
predictive control strategy is proposed for the analge-
sia process based on a two-compartmental fractional
derivative model. Simulations show the effect of the
fractional order on the concentration of Propofol in
a patient. We conclude that different values for the
fractional order of the model can capture inter-patient
variability, making compartmental fractional deriva-
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
100

tive models suitable to model the diffusion process in
the human body.
ACKNOWLEDGEMENTS
C. M. Ionescu acknowledges the Flanders Research
Center (FWO) for its financial support. This re-
search is supported by Flemish Research Foundation
-Research Project FWOPR2013 005101.
REFERENCES
Absalom, A. R., De Keyser, R., and Struys, M. M. (2011).
Closed loop anaesthesia: are we getting close to
finding the holy grail? Anesthesia & Analgesia,
112(3):516–518.
Beneken, J. E. and van Oostrom, J. H. (1998). Modeling in
anesthesia. Journal of Clinical Monitoring and Com-
puting, 14:57–67.
De Keyser, R. (2003). A gentle approach to predictive con-
trol. UNESCO Encyclopaedia of Life Support Systems
(Eolss).
De Keyser, R. and Van Cauwenberghe, A. (1981). A self-
tuning multisteppredictor application. Automatica,
17:167–174.
D’hulster, F., De Keyser, R., and Van Cauwenberghe, A.
(1983). Aplication of adaptive controllers to a paper
machine headbox. Automatica, 19(4):407–414.
Dokomuetzidis, A., Magin, R., and Machetas, P. (2010).
Fractional kinetics in multi-compartmental systems.
Journal of Pharmacokinetic and Pharmacodynamic,
37:507–524.
Garcia, P. S., Kolesky, S. E., and Jenkins, A. (2010). Gen-
eral anesthetic actions on gaba receptors. Curr Neu-
ropharmacol, 8(1):2–9.
Hemmerling, T. M.and Salhab, E., Aoun, G., Charabati,
S., and Mathieu, P. (2007). The ’analgoscore’: a
novel score to monitor intraoperative pain and itsuse
for remifentanil closed loop application. IEEE Inter-
national Conference on Systems, Man and Cybernet-
ics, pages 1494–1499.
Keele, C. A. (1970). Chemcial causes of pain and itch. An-
nual Review of Medicine, 21:67–74.
Kuizenga, K., Proost, J. H., Wierda, J. M., and
Kalkman, C. J. (2001). Predictability of pro-
cessed electroencephalography effects on the basis of
pharmacokinetic-pharmacodynamic modeling during
repeated propofol infusions in patients with extradu-
ral analgesia. Anesthesiology, 95:607–615.
Magin, R. L. (2010). Fractional calculus models of complex
dynamics in biological tissues. Computers & Mathe-
matics with Applications, 59(5):1586–1593.
Marieb, E. N. and Hoehn, K. (2011). Anatomy & Physiol-
ogy. Pearson Education, 4 edition.
Melzack, R. (2011). The story of pain. Psychologist,
24(6):470–471.
Popovic, J. K., Atanackovic, M. T., Rapaic, A. S., Pilipovic,
S., and Atanackovic, T. M. (2010). A new approach to
the compartmental analysis in pharmacokinetics: frac-
tional time evolution of diclofenac. Journal of Phar-
macokinetic and Pharmacodynamic, 37:119–134.
Wagner, J. G. (1976). Linear pharmacokinetic equations
allowing direct calculation of many needed pharma-
cokinetic parameters from the coefficients and expo-
nents of polyexponential equations which have been
fitted to the data. Journal of Pharmacokinetics and
Biopharmaceutics, 4(5):443–467.
West, B. J. (1990). Fractal physiology and chaos in
medicine, Studies of nonlinear phenomena in life sci-
ences, volume 1. World Scientific.
Zwart, A., Smith, N., and Beneken, E. (1972). Mul-
tiple model approach to uptake and distribution of
halothane - the use of an analog computer. Computers
and Biomedical Research, 5:228–238.
Non-invasivePainSensorDevelopmentforAdvancedControlStrategyof
Anesthesia-AConceptualStudy
101
