
Towards a Personalised Health System
Peter Weller
1
, Alberto Fernández Gil
2
and Eduardo Alsonso
3
1
Centre for Health Informatics, City University London, London, U.K.
2
CETINIA, University Rey Juan Carlos, Madrid, Spain
3
Department of Computer Science, City University London, London, U.K.
Keywords: Clinical Decision Support Systems, Personalised Healthcare, Software Agents.
Abstract: This paper presents a Personalized Healthcare System (PHS), a decision support tool that can adapt to
changing conditions, such as aging and illness, in individual patients. The system consists of three
components: a unique personalised profile, a collection of web based tools and a web based repository for
managing interactions between clinicians and tools. The proposed system makes extensive use of software
agents, both for collecting the initial information required to construct a personalized profile and for
transporting the information needed to use the on-line decision support tools. The paper discusses the
operation of a PHS and suggests possible implementation issues.
1 INTRODUCTION
This paper introduces the concept of a Personalised
Healthcare System (PHS). It discusses basic
components, describes a number of functions of a
PHS and offers some suggestions on
implementation.
A PHS is a software-based system that is unique
for every member of a population. Depending on the
patient, on their current health status and location, it
could either be running continuously (as a semi-
active or active Decision Support Sys-tem (DSS)) or
activated only when required (as a passive DSS). A
PHS changes and evolves over the life of the
associated person by adapting to events such as
aging, illness, accidents and life-style choices.
Furthermore, it can link with the PHS of family
members, friends and neighbours to gain knowledge
of hereditary or location-based diseases or illnesses.
The purpose of the PHS is to support the care
and treatment of individual patients in a variety of
situations, primary and secondary care, and
telehealth via tele-consulting. The system includes
access to both diagnosis tools for assessing current
health status and prediction tools to consider “what-
if” scenarios for the effect of treatments and life-
style changes.
Healthcare has been one of the target application
areas of expert decision support systems for many
years, including recent distributed technologies such
as multi-agent systems (Paranjape and Sadanand,
2009 & Shirabad et al., 2012), and SOA (Nadkarni
and Miller, 2007). However, to the best of our
knowledge, frameworks supporting the integration
of different DSS and generic profile knowledge for
health care has not been addressed by previous
works.
2 STRUCTURE OF PHS
The PHS is composed of three main components:
1) A client program, which constructs a Personal
Patient Profile (P3) and co-ordinates the
information flow process for each patient;
2) A central web-based repository that stores basic
pro-files and manages interactions between
clients and remote tools.
3) A collection of remote web-based tools for DSS
and modelling/prediction tasks;
2.1 Client Program
A core task of the client program is to construct the
P3, a key component of the PHS. The P3 is
constructed prior to any consultation from three
resources, namely, the patient’s Electronic Patient
Record (EPR) EPR, a generic patient profile, and the
latest information from external data sources such as
news links, journals and social media sites. The
256
Weller P., Fernández Gil A. and Alonso E..
Towards a Personalised Health System.
DOI: 10.5220/0004749702560261
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2014), pages 256-261
ISBN: 978-989-758-010-9
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

client program also contains the mechanisms for
sending requests via soft-ware agents and receiving
information back from other soft-ware agents.
2.2 Central Repository
The central repository contains four elements:
1) A library of generic profiles for a range of
patient scenarios;
2) A database of decision support tools;
3) A database of modelling tools;
4) A suite of management tools for organising and
up-dating the previous elements.
Generic Profiles
The library of generic profiles consists of sets of
data relevant to different patient scenarios, ages and
conditions, for example, a four-year-old girl with
diabetes or a fifty-year-old man with high blood
pressure.
The base profile used will change during the
patient’s life. When a baby is born it is given a
profile for a new-born baby. A range of these will be
available for different initial conditions such as
premature births, multiple births or birth
complications. Some facts in the initial profile could
be obtained from the parents’ and siblings’ EPR. As
the baby ages, the profile changes to include
expected conditions such as measles and
chickenpox, and developmental features, for
example, teething, walking and the beginnings of
speech. If a condition or feature is no longer relevant
to the baby it is not included, so milk teeth will not
appear in a profile for a fifteen-year-old male. Over
time, the requirements for making decisions related
to baby health are replaced by those associated with
young children. These, in turn, are superseded by
those for older children and then for adolescents,
young adults, etc. In addition, details for illnesses,
accidents and events not generally considered part of
the basic health scenario, for example the
development of diabetes, are added as required.
Eventually, the profile will contain information
associated with aging and geriatric conditions, such
as dementia, rheumatism or senility. Finally when a
person dies the PHS is retained as a source of
information for descendents, friends and neighbours.
New users joining the system could be given an
initial profile based on sex, age and basic health.
Database of Decision Support Tools
This database contains the details of all decision-
support tools registered on the system. The
information stored includes: a brief description of
the application domain; input data required for
operation; cost of service (if applicable); a measure
of accuracy and expected results from the developer
(in terms of sensitivity/specificity), and finally
feedback for users both as a rating and as text.
Database of Modelling Tools
Following the same approach as the one outlined
above, this database stores details of modelling and
prediction tools stored in the system.
Suite of Management Tools
The management tools perform two tasks: sending
and receiving information via intelligent agents, and
the maintenance of the database of third party
decision support and modelling tools.
2.3 Web based Tools
Decision Support Systems
This component of the system consists of a suite of
individual DSSs that are developed and maintained
by independent third parties (e.g., universities,
charities or disease specific research groups). They
are registered with the PHS central repository and
made available via the internet. Each module could
be developed for a specific function, for example the
diagnosis of an illness or condition. These modules
would be constructed using a variety of data
analysis, decision-making or prediction tools
selected by the developers.
Modelling Tools
Similarly, this component consists of a suite of
modelling tools (MTs) for predicting a range of
scenarios. They are also developed and maintained
by third parties and registered on the PHS central
repository.
3 OPERATION OF A PHS
The following scenario illustrates a typical exchange
between a GP and a patient, although the
methodology would be very similar for a clinician
caring for a patient in a hospital. Before each
consultation, once the patient confirms their
attendance, the client program constructs the
personalised patient profile (P
3
). To do this Agent A
is sent to the Central Repository with brief patient
details. Agent B is returned with the most
appropriate generic profile. This profile is then
integrated with the patient’s EPR and any relevant
news items or pertinent issues from social media
resources. Finally, intelligent agents are used to
conduct an on-line search for any relevant new
TowardsaPersonalisedHealthSystem
257
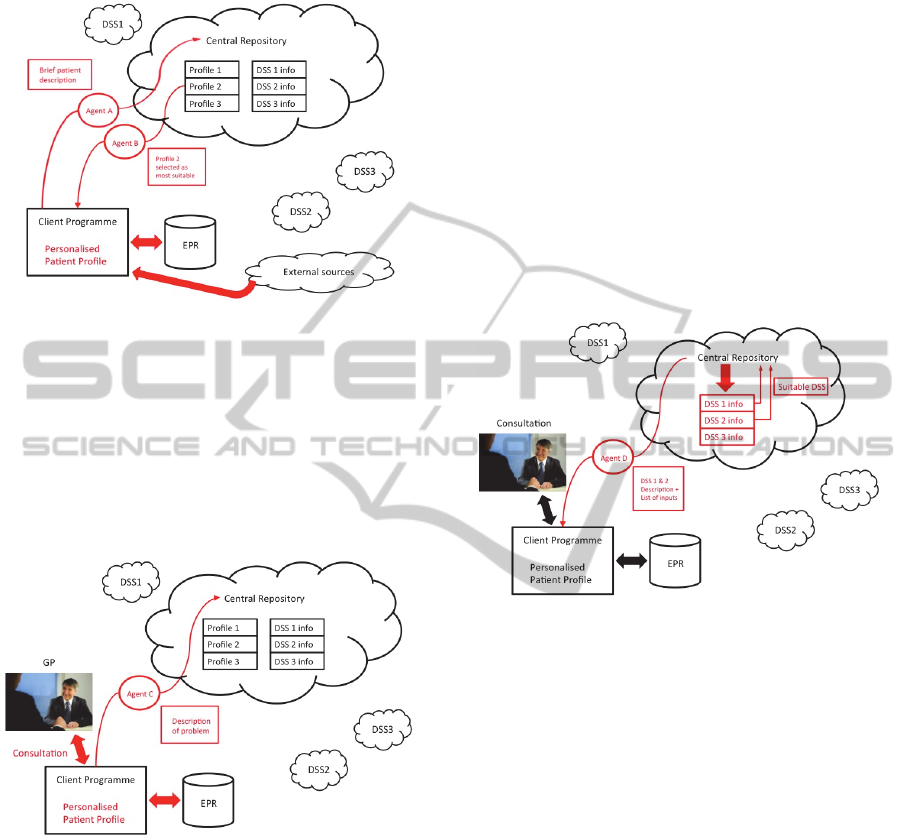
treatments and evidence-based guidelines. These
steps are shown in Figure 1.
Figure 1: Selection of Profile and construction of P
3
.
Figure 2 depicts the initial situation, with a
patient meeting with their GP. The GP starts to
assess the patient’s condition with a set of questions.
During the consultation the patient’s symptoms and
responses are added to the P
3
(and into the EPR) via
speech recognition or typing. These inputs are used
to refine any search strategy to provide more
relevant and more recent guidance.
Figure 2: Meeting with GP and request for DSS support.
From the information and data collected during
an initial dialogue a preliminary diagnosis would be
made. In addition, central resources may need to be
consulted for advice or clarification. In order to do
so, the program despatches Agent C, loaded with
some basic individual information extracted from
the EPR and from the consultation (for example,
fifty-year-old male, chest pains, hypotension) to the
central repository. This stage is shown in Figure 2.
The central repository now matches the
information supplied to a list of available DSSs
using the descriptors provided by the DSS
developers. Not all DSSs will be relevant to the
current problem. Only those that match the problem
details (to a greater or lesser extent) are returned to
the co-ordinator with a list of the inputs and
information required for the DSS, such as a feedback
rating. This situation is illustrated in Figure 3, where
two DSSs (DSS 1 and DSS 2) are identified as
possibly being suitable for the requested task. This
does not mean that each identified DSS is
necessarily able to perform the required diagnosis,
just that there is some match between the problem
and the capabilities of the DSS. For example, DSS 1
may have been developed for diagnosing tumours in
a fifty-year-old male, whereas DSS 2 may have been
developed for diagnosing a heart attack. The
information collected by the coordinator is sent to
the clinician via Agent D.
Figure 3: Selection of possible DSS and dispatch of details
about suitable DSS.
The clinician now decides which DSS is most
capable of diagnosing the patient’s condition. This
could be based on a number of criteria, including
relevance of problem domain, data required as
inputs for the DSS and even a ranking based on
previous performance. There could be a cost
function associated with using a DSS, for example a
privately developed DSS could be made available
but with a charge levied each time it is selected. In
Figure 4, DSS 2 has been selected and Agent E is
sent, with the required data, to the central repository
in order for the diagnosis to be reached.
The central coordinator then sends Agent F with
the data to DSS 2. This approach maintains
confidentiality and ensures that no patient
identifiable information is sent to the DSS. This
stage is shown in Figure 5.
Once the diagnosis has been reached an agent is
sent back to the central coordinator with the output.
The data is also added to the DSS knowledge-base
for further development of the tools. In Figure 5,
Agent G has been dispatched by the Central
HEALTHINF2014-InternationalConferenceonHealthInformatics
258
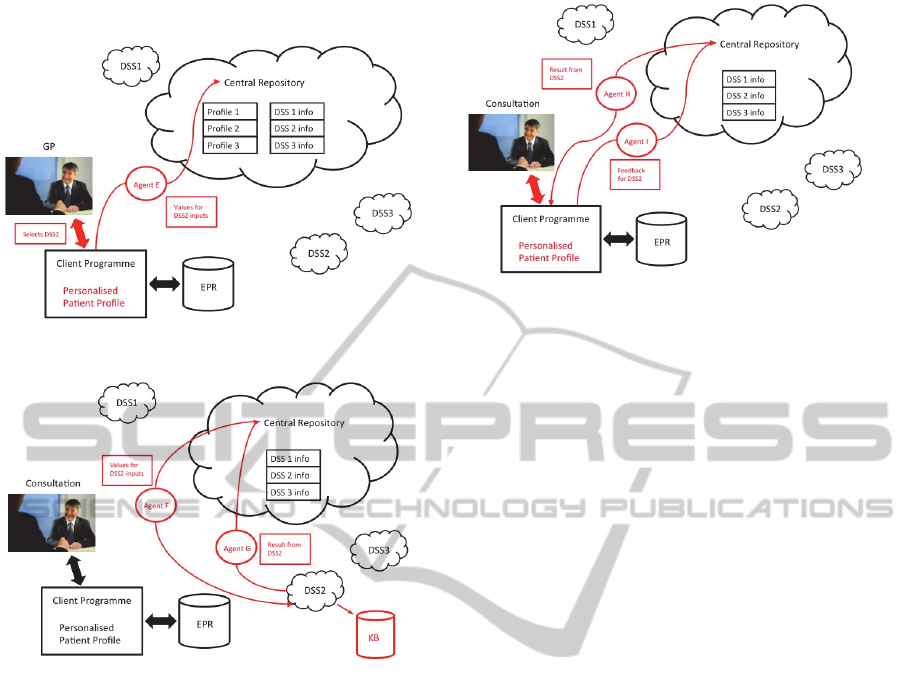
Repository with the output from DSS 2.
Figure 4: Selection of DSS and data for diagnostic request.
Figure 5: Transmission of data to selected DSS and
transmission of DSS result.
The central coordinator then transmits the result
to the GP through Agent H, as illustrated in Figure 6.
This information can be added to the patient’s EPR
for future reference. Finally the Client Program
sends Agent I with the GP’s feedback on the
performance of DSS2. This is added to the central
register for use with future requests.
The figures above only show the main tasks.
Additional support-functions such as receipt of
information, safe transmission and encryption are
not included but would be components of the
process nevertheless. A similar approach would be
adopted for modelling the effects of a treatment or
drug regime.
4 SYSTEM IMPLEMENTATION
In this section we present some ideas on the
implementation of the PHS. We consider service-
oriented architectures (SOA) and ontologies as the
enabling technologies towards the implementation of
Figure 6: Transmission of DSS output and feedback.
this kind of systems.
SOAs (Huhns and Singh, 2005) comprise at least
two components: service providers and service
clients. Services are software components that
encapsulate some functionality. Providers and
clients interact for some service to be carried out.
Basically, the client provides the inputs to the
service (e.g., patient symptoms), and the provider
returns the results/outputs of the execution of the
service (e.g., diagnostic). Typically, a third
component, a service registry (also known as a
directory) is also present. Providers advertise their
services by registering a description with the
directory. An example of information included in a
service description can be the type of inputs
expected and of outputs provided. Service
descriptions can be specified at different levels of
expressivity ranging from purely syntactic to
complex logic-based descriptions (Fernandez et al.,
2012). An ontology is a specification of a
conceptualization (Gruber, 1993). Ontologies are
used to share information/knowledge, or more
specifically to share the vocabulary used when
agents/services interact. For example, it is important
that the concept “blood pressure” is shared, and then
understood the same way (e.g. units), both by the GP
and the diagnosis tool.
Ontologies are used in PHS for knowledge
representation such as generic profiles, EPR, P
3
,
DSS/modelling tool descriptions and communication
message contents.
Figure 7 depicts the main building blocks of the
PHS architecture. As presented in previous sections,
the PHS is composed of three main elements, a
client program, a set of remote tools and a central
repository. We propose the use of a service-oriented
architecture (SOA) to coordinate the interaction
among the different actors in our PHS.
TowardsaPersonalisedHealthSystem
259
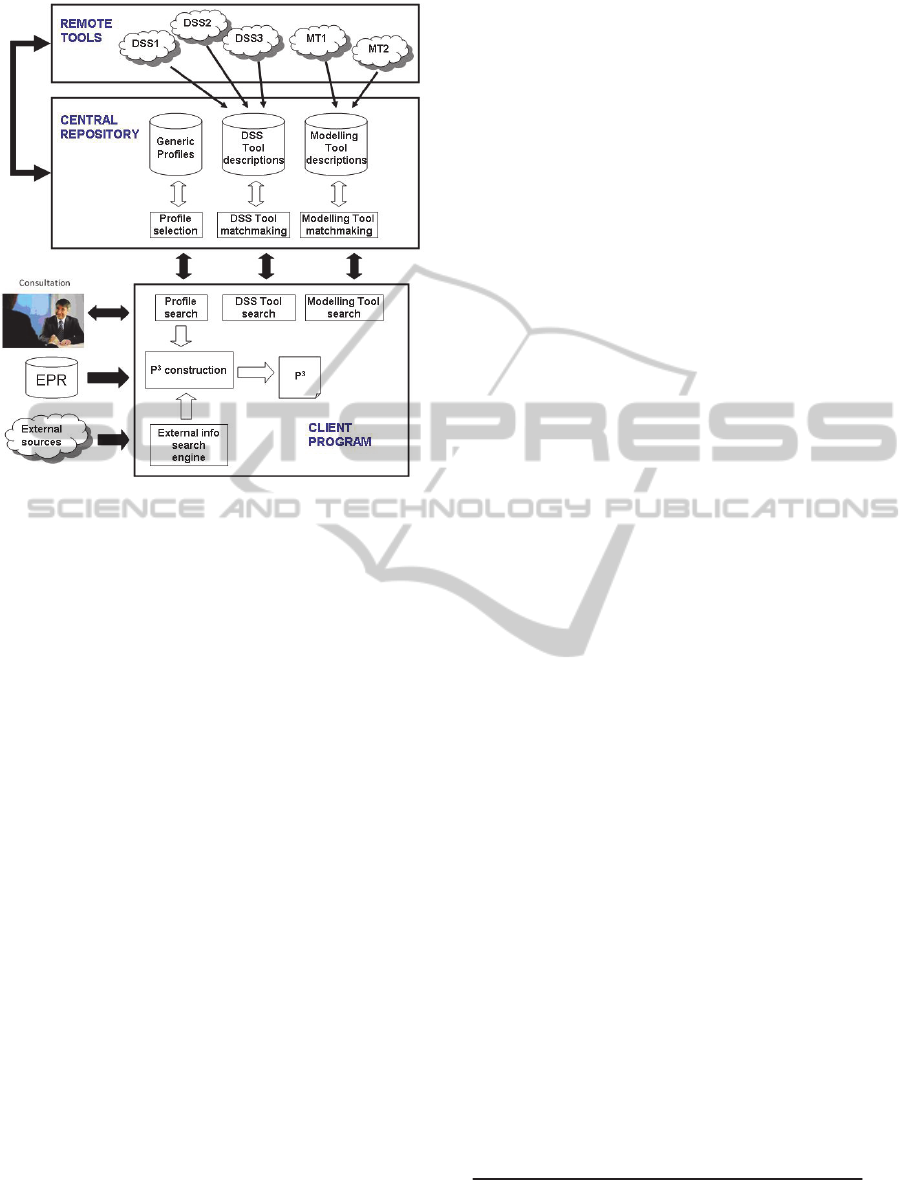
Figure 7: PHS architecture.
In the rest of this section some details about each
component are given.
4.1 Remote Tools
DSS and modelling tools in the PHS are represented
as (web) services in our SOA. Providers advertise
their services by registering a description of their
tools with the Central Repository. The set of existing
DSS and MTs is not fixed and can vary over time. In
fact, it is natural that new tools appear as time goes.
Tools are created and maintained by third parties.
Different tools may differ on their specialty,
generality/specificity, technique, cost, etc. Several
tools might target the same objective (e.g. disease)
but be provided by different institutions/companies.
In some cases, aspects such as cost, trust and
reputation might have to be considered so as to
select the appropriate provider. The proposed SOA
architecture gives flexibility to developers to add,
modify or remove tools. They only have to register
the description with the Central Repository.
4.2 The Central Repository
The main functionality of the Central Repository is
to store and manage basic patient profiles and
remote tools. Thus, it contains three databases,
namely generic profiles, DSS tool descriptions and
modelling tool descriptions.
Each generic profile comprises two elements, a
profile pattern description (e.g. four-year-old girl
with diabetes) and the profile detailed information.
Profile descriptions are used to identify the adequate
profile according to the characteristics of the patient
provided by the GP through the Client Program.
This task is carried out by the Profile selection
module. Concept similarity (Euzenat and Shvaiko,
2007 & Fernandez et al., 2007) techniques can be
applied for this task.
The Central Repository also provides
matchmaking functionalities to locate DSS and
modelling tools (DSS and Modelling Tool
matchmaking modules in Figure 13). Tool providers
register a (semantic) description of their DSS tool
(e.g., diagnosis of lung cancer in teenagers) with the
Central Repository. When the GP (or just a user)
decides to consult a DSS they specify a description
of the desired service. The Central Repository then
matches the request against the registered tools and
returns the information about the ones appropriate
for that task. Descriptions for these tools are more
complex than the ones for patient profiles so
advanced methods will be used here. In particular,
techniques from the semantic web service
matchmaking (Klusch, 2008) will be adapted and
applied for the specific particularities of DSS and
modelling tool discovery.
Note that, although conceptually centralized, a
distributed implementation could be adopted for
efficiency or fault tolerance. For example, three
different repositories each containing a database and
its corresponding matchmaking functionality would
be a straightforward distribution, but others would
be also possible.
4.3 The Client Program
The Client Program (CP) is in charge of mediating
between the GP and the rest of the system, which
includes the construction of the P
3
as well as the
interaction with the Central Repository to find
generic profiles, DSS or modelling tools. The
construction of the P
3
is the main and more complex
task carried out by the CP. As previously described,
it requires the EPR, a generic patient profile, and
information from external data sources. Ontologies
have been proposed as a technique for automatic
processing and interoperating healthcare
representation standards like SNOMED CT
1
, HL7
2
,
OpenEHR
3
or CEN 13606
4
(Gomez-Perez et al.,
1
www.snomed.org
2
http://www.hl7.org/
3
http://www.openehr.org/
4
http://www.cen.eu/
HEALTHINF2014-InternationalConferenceonHealthInformatics
260

2009; Sahay et al., 2012 & Schloeffel et al., 2006).
The External info search engine module is in
charge of accessing external information sources
(databases, journals, social media, etc.). Ad-hoc
wrappers might be needed for different sources. We
believe that the increasing adoption of Linked Data
5
as a standard way of exposing information will ease
the integration of the different information sources.
In fact, there are already several related linked data
sources such as DailyMed
6
or MediCare
7
, which
provide information about existing drugs.
5 CONCLUSIONS
In this paper we have proposed a Personalised
Healthcare System (PHS). Key characteristics of
such a system are its uniqueness for every member
of a population; its capacity to evolve over the life of
the person; and its flexibility to coordinate external
resources such as DSS/modelling tools and
information sources. We described the operation of a
PHS and gave some ideas towards its
implementation following a SOA approach.
It is part of our future work the implementation
of a PHS as well as extending the architecture
improving aspects such as mechanisms for tool
selection (e.g. trust and reputation, auctions, etc.).
ACKNOWLEDGEMENTS
Work supported by the Spanish Government, grants
TIN2009-13839-C03-02, CSD2007-0022 and
TIN2012-36586-C03-02).
REFERENCES
Euzenat, J., Shvaiko, P., 2007. Ontology matching.
Springer, Heidelberg (DE).
Fernandez, A., Cong, Z., Balta, A., 2012. Bridging the
Gap Between Service Description Models in Service
Matchmaking. Multiagent and Grid Systems, 8 (1): 83-
103.
Fernandez, A., Polleres, A., Ossowski, S., 2007. Towards
Fine-grained Service Matchmaking by Using Concept
Similarity. Workshop on Service Matchmaking and
Resource Retrieval in the Semantic Web (SMR2 at
ISWC), pp. 31-45.
Gomez-Perez, J. M., Kohler, S., Melero, R., Serrano-
5
http://linkeddata.org/
6
http://www4.wiwiss.fu-berlin.de/dailymed/
7
http://www4.wiwiss.fu-berlin.de/medicare/
Balazote, P., Lezcano, L., Sicilia, M.A., Iglesias, A.,
Castro, E., Rubio, M., Buenaga, M., 2009. Towards
Interoperability in e-Health Systems. International
Conference on Health Informatics: 205-210.
Gruber, T. R., 1993. A translation approach to portable
ontologies. Knowledge Acquisition, 5(2): 199-220.
Huhns, M. N., Singh, M. P., 2005. Service-Oriented
Computing: Key Concepts and Principles. IEEE
Internet Computing, 9(1):75–81.
Klusch, M., 2008. Semantic Service Coordination. In
CASCOM - Intelligent Service Coordination in the
Semantic Web. Birkhaeuser Verlag, Springer: 59-104.
Nadkarni, P. M., Miller, R. A., 2007. Service-oriented
Architecture in Medical Software: Promises and
Perils. Journal of the American Medical Informatics
Association, 14 (2), pp 244-246.
Paranjape, R., Sadanand, A., 2009. Multi-Agent Systems
for Healthcare Simulation and Modeling: Applications
for System Improvement. ISI Global.
Sahay, R., Zimmermann, A., Fox, R., Polleres, A.,
Hauswirth, M., 2013. A Formal Investigation of
Semantic Interoperability of HCLS Systems.
Interoperability in Healthcare Information Systems:
Standards, Management, and Technology. IGI Global.
Schloeffel, P., Beale, T., Hayworth, G., Heard, S., Leslie,
H., 2006. The relationship between CEN 13606, HL7,
and openEHR. In Health Informatics Conference,
Sidney, Australia.
Shirabad, J. S., Wilk, S., Michalowski, W., Farion, K.,
2012. Implementing an Integrative Multi-agent
Clinical Decision Support System with Open Source
Software. Journal of Medical Systems, 36 (1), pp 123-
137.
TowardsaPersonalisedHealthSystem
261
