
Modelling of Genetic Interactions in GWAS Reveals More Complex
Relations between Genotype and Phenotype
Joanna Zyla
1
, Christophe Badie
2
, Ghazi Alsbeih
3
and Joanna Polanska
1
1
Data Mining Group, Faculty of Automatic Control, Electronic and Computer Science, Silesian University of Technology,
Akademicka 16, Gliwice, Poland
2
Cancer Genetics and Cytogenetics Group, Biological Effects Department, Centre for Radiation,
Chemical and Environmental Hazards, Public Health England, Didcot, OX11 ORQ, U.K.
3
Radiation Biology Section, Biomedical Physics Dept., King Faisal Specialist Hospital & Research Centre,
Riyadh 11211, Saudi Arabia
Keywords:
GWAS, Radiosensitivity, Polymorphism, Data Mining.
Abstract:
The aim of this work is to present the complete methodology useful in GWAS analysis with small sample size,
where comprehension of interaction between the genotype and phenotype is a main issue. By including all
possible models of interaction into the process of model building, we were able to significantly increase the
number of candidate polymorphisms and decrease the false discovery ratio.
1 INTRODUCTION
Every day our body is exposed to different types of
radiation, which we can divided into two groups: ion-
izing radiation (IR) and non-ionizing one (nIR). Be-
cause of their high energy, all ionizing radiation cause
the DNA damage in cells, while non-ionizing radia-
tion doesn’t damage DNA directly, affecting cells in
other ways (Wrixon et al., 2004) (UNSCEAR, 2010).
The ionizing radiation is one of the leading treatment
of cancer though it causes many sides effects to the
patient (Burnet et al., 2006) (NCI, 2012). People re-
act to IR in different ways, some of them can stand
very aggressive radiotherapy, while the others demon-
strate high radio-intoxication just after the start of the
treatment. This is why investigation of the individual
reaction to ionizing radiation is so important to im-
prove the overall health care level, to minimize the
cost of the treatment and, what is the most important,
to increase the patient comfort.
The reaction to IR is called radiosensitivity, which
is defined as individual ability of cells, tissues, or-
gans or organisms to the deal with the harmful effect
of ionizing radiation. In 1906, Bergonie and Tribon-
deau find out that the radiosensitivity of cells is di-
rectly proportional to their activity and inversely pro-
portional to the degree of differentiation (Bergoni and
Tribondeau, 1906). Radiosensitivity has been shown
to be heritable (Roberts et al., 1999). The investiga-
tion of biomarkers to asses the radiosensitivity is one
of the leading problem in radiation biology. The most
prominent biomarker is the activity of phosphoryla-
tion of histon H2AX, which gives a starting signal
to double strand break repair (Taneja et al., 2004).
However, it does not explain the observed diversity
of radiosensitivity among patients, and there is still a
big demand for search of new biomarkers. Main goal
of this study is to demonstrate that proper techniques
used to analyse the experimental data might lead to an
extra discoveries by significant increase in the number
of candidate biomarkers.
One of the way of polymorphism impact investi-
gation is Genome-Wide Association Study (GWAS),
which seems to be very efficient in search for genetic
background of common diseases or phenomena like
radiosensitivity. The first GWAS results were pub-
lished in 2005 (Klein et al., 2005). The number of
GWAS has been increasing since that, and, as it is
stated in National Human Genome Research Institute
database, for the day 25th of August 2013 there were
1716 GWAS-based publications, which report 11586
polymorphisms associated with diseases under inves-
tigation. The definition of GWAS says that the associ-
ated polymorphisms should be searched by genotyp-
ing at least 100,000 of them in the possible large pop-
ulation (Hindorff et al., 2009). In case of binary out-
come, the most popular methods to detect and assess
the risk alleles are the logistic regression and contin-
204
Zyla J., Badie C., Alsbeih G. and Polanska J..
Modelling of Genetic Interactions in GWAS Reveals More Complex Relations between Genotype and Phenotype.
DOI: 10.5220/0004807402040208
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2014), pages 204-208
ISBN: 978-989-758-012-3
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
gency tables (Bush and Moore, 2012)(Kamboh et al.,
2012) (Chung et al., 2013). In case of quantitative
trait tests like ANOVA are used. Because of the series
of statistical test being performed, correction for mul-
tiple testing is necessary (Lin and Lee, 2012) (Pahl
and Schafer, 2010).
In radiosensitivity the most important finding
based on GWAS were published by Niu at el, where
they show five genes (C13orf34, MALD2L1, PLK4,
TPD52, and DEPDC1B) validated by siRNA knock-
down experiment (Niu et al., 2010) and by Lin at el.
which shows 12 significant polymorphisms related to
the response to radiation by using the radiation hy-
bryd network (Lin et al., 2005).
2 MATERIALS AND METHODS
2.1 Subject
The population under investigation is composed of 44
unrelated Caucasian individuals (uR).
2.2 Biological Experiments
Two types of information were collected per each par-
ticipant. First one was the result of genotyping of
567,096 polymorphisms by Axiom myDesign arrays.
Number of genotyped SNPs at each chromosome is
presented in Table 1.
Table 1: Number of analysed polymorphisms by chromo-
somes.
Chr. # of SNPs Chr. # of SNPs
1 41606 14 18302
2 45191 15 17030
3 39829 16 16376
4 37097 17 11655
5 35598 18 17749
6 43140 19 6899
7 30713 20 13297
8 31653 21 7894
9 26212 22 5523
10 27762 X 15790
11 25821 Y 2025
12 27262 MT 273
13 22397
TOTAL 567096
The second dataset includes activity of H2AX
measured in two experimental conditions: 1) just af-
ter the irradiation of 2Gy and 2) in normal condi-
tions. The irradiations were performed at room tem-
perature with an A.G.O. HS X-ray system (Aldermas-
ton, Reading, UK) (output 13 mA, 250 kV peak, 0.5
Gy/min for doses 0.5 4 Gy and 0.2 mA 4.9 mGy/min
for doses up to 100 mGy). As the group o cells the T-
lymphocyte cultures were prepared using the method
described previously (O’Donovan et al., 1995; Finnon
et al., 2008).
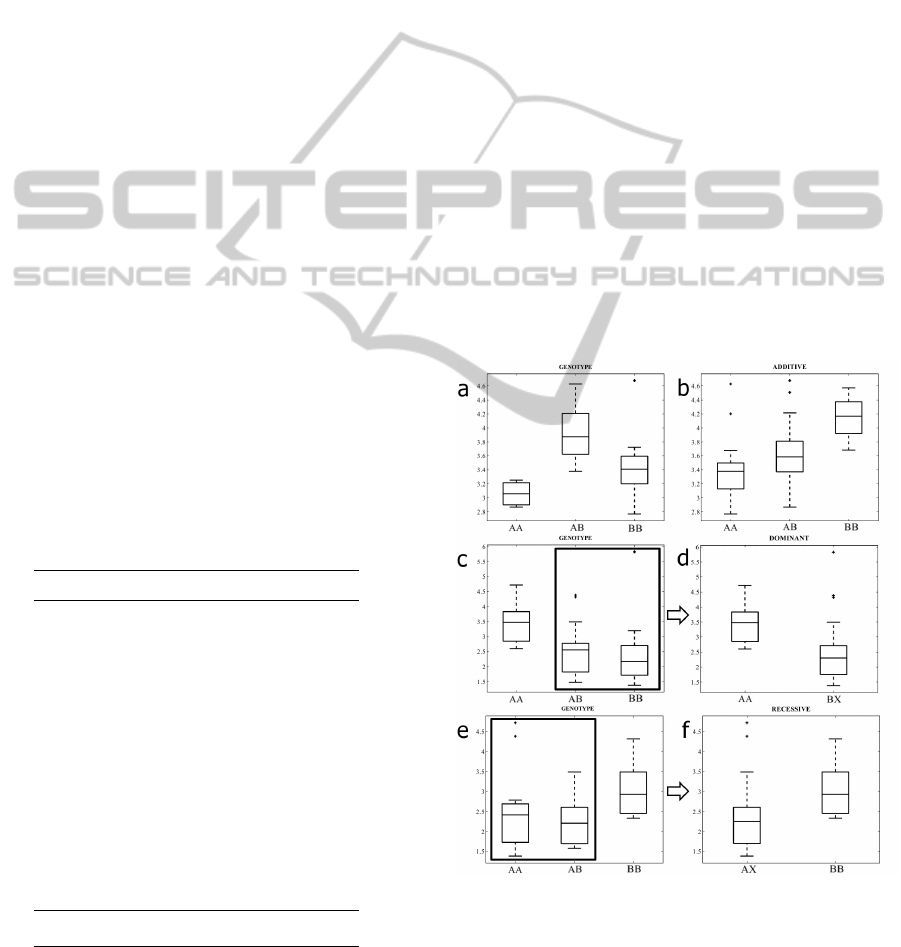
2.3 Models of Interaction
Three different models of SNP-gene expression inter-
action were investigated in this study. First of them
is a genotype model, where each of the alleles gives
independent level of gene expression (see Fig.1a).
There is a special case of such association, called ad-
ditive model, where each additional copy of the vari-
ant allele increases/decreases the response (Fig.1b).
Another interaction model analysed in this study is a
dominant one, where single variant allele impacts the
gene expression level the same way as double vari-
ant allele form (Strachan and Read, 1999) (Fig.1c).
The last model we distinguish is a recessive model,
where only two variant alleles have impact on expres-
sion profile (Lewis, 2002) (Fig.1e).
Figure 1: The boxplots of H2AX signal value presented ac-
cording to the type of interaction model. Panel a) standard
genotype interaction; b) additive interaction; c) boxplot of
signal distibution in dominant interaction; d) final dominant
model; e) boxplot of signal distibution in recessive interac-
tion; f) final recessive model
ModellingofGeneticInteractionsinGWASRevealsMoreComplex
RelationsbetweenGenotypeandPhenotype
205

2.4 Statistical Analysis
Since the measurements of H2AX phosphorylation
were taken in two experimental conditions, the
normalized values of signal induction after IR were
calculated with the use of reference genes and all of
the analyses were performed at two endpoints: 0Gy
and logarithm of fold change (FCH). The normality
of signal distribution was verified by Lilliefors test.
Homogeneity of variance was checked by Bartlett’s
test for genotype model, and F test for dominant and
recessive models. The parametric tests for equiva-
lence of population mean values were used (ANOVA
for genotype, t-test for dominant and recessive
models). In the next step, the best interaction model
per each polymorphism at every endpoint was chosen
with the use of minimum p-value criterion. Per every
SNP, the minimum p-value was chosen from the set
of three p-values obtained for the analysed models
of interaction: genotype (p
G
), dominant (p
D
), and
recessive (p
R
) - Figure 2.
Figure 2: Algorithm of decision making on the final model
of interaction for particular SNP at chosen endpoint with
the use of minimum p-value critertion.
As a set of candidate polymorphisms those with
minimum p-value<0.05 were considered. The false
discovery ratio was estimated to accompany set of
candidate polymorphisms.
3 RESULTS AND DISCUSSION
Table 2 presents the summary of genotyped data. The
results of normality testing presents table 3.
One can observe that the number of null hypoth-
esis rejections is lower than the expected by chance,
which leads to the general acceptance of null hypoth-
esis on normality of signal distribution. While look-
ing at the results of the verification of hypothesis on
homogeneity of variances (see table 4), the observed
number of hypothesis rejections exceeds the expected
Table 2: Summary of polymorphism genotyping in group
under investigation.
Genotyping results # of SNPs %
single form only 33,679 5.93%
two different forms 235,007 41.44%
all three forms 147,413 25.99%
Table 3: Results of testing on normality of signal distribu-
tion.
Model of interaction
Genotype Dominant Recessive
# of tests 442,245 558,028 636,418
0Gy
p<0.05
N 13,502 11,262 12,686
% 3.05 2.02 1.99
FDR 100 100 100
FCH
p<0.05
N 19,545 17,637 20,274
% 4.42 3.16 3.19
FDR 100 100 100
Table 4: Results of testing on homogeneity of variances.
Model of interaction
Genotype Dominant Recessive
Total 147,415 279,014 318,209
0Gy
p<0.05
N 5,354 11,130 12,837
% 3.63 3.98 4.03
FDR 100 100 100
FCH
p<0.05
N 8,107 16,199 18,537
% 5.5 5.81 5.83
FDR 100 86.12 85.83
Table 5: The results of comparison study of H2AX at both
endpoints - 0Gy and FCH.
Model of interaction
Genotype Dominant Recessive
Total 147,415 279,014 318,209
0Gy
p<0.05
N 6,947 26,668 30,641
% 4.71 9.56 9.63
FDR 100.00 52.31 51.93
FCH
p<0.05
N 7,177 27,675 31,579
% 4.86 9.92 9.92
FDR 100.00 50.41 50.38
by chance, which forces the use of proper parametric
tests with correction for unequality of variances.
Finally, the hypothesis on population mean value
equality was checked, separate analyses were per-
formed for all three models of interactions. The sum-
mary of these is presented in table 5.
The criterion of minimal p-value was used to get
the final model for particular SNP-endpoint interac-
tion, the results are presented in table 6. In tested his-
BIOINFORMATICS2014-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
206

ton phosphorylation activity majority of SNPs repre-
sent dominant or recessive model, and very few (with
high FDR) represent genotype interaction.
Table 6: The results of the final optimal model selection for
H2AX at both endpoints - 0Gy and FCH.
Optimal model of interaction
Genotype Dominant Recessive
0Gy
N 1,159 25,052 29,078
FDR 100 55.69 54.72
FCH
Nl 1,050 26,040 29,901
FDR 100 53.57 53.21
All the polymorphisms with minimal p-value for
final model being less than 0.05 were considered as
the candidate SNPs related to the radiosensitivity phe-
nomena. If genotype model was only considered,
there would be 1159 candidate SNPs for 0Gy and
1050 candidate SNPs for FCH found with FDR equal
to 100%. If additional models of interaction, reces-
sive and dominant, are included into the study to-
gether with the proposed method for model selec-
tion, the number of candidate SNPs increases signif-
icantly to 55,289 giving FDR at the level of 67.34%.
Only 2.1% of final models are genotype interactions
at 0Gy and 1.84% for FCH endpoint, while 45.31%
and 45.69% respectively are of dominant type. The
most frequent for both endpoints is recessive model
with 52.59% at 0Gy and 52.46% for FCH of candi-
date SNPs.
The filtration of candidate SNPs could be done in
several ways, some might apply the standard statisti-
cal corrections for multiple testing, another might go
for candidate SNP validation done with independent
sample, the others might validate SNPs by the func-
tional analysis. Due to the limited power of statistical
tests, resulting from relatively small sample size, the
application of multiple testing correction techniques
is not recommended. We do propose to check on
their genome location and functional class. Since the
nonsynonymous SNPs (nSNP), are the most interest-
ing because they directly affect translated amino-acid
protein sequence (Ramensky et al., 2002), the SNP
validation might be done by requiring from candidate
SNP to be nSNP. The average number of nonsynony-
mous SNPs is 1.32% and 1.23% (for 0Gy and FCH
endpoints respectively), and the average percentage
of polymorphisms in functional regions (intron, exon,
nsSNP, UTR etc.) is equal to 41.36% and 41.27% re-
spectively, table 7.
Table 7: Functional description of obtained candidate SNPs.
# SNPs
# Functional
SNPs (%)
0Gy 55,289 22,868 41.36
FCH 56,991 23,519 41.27
intersection 7,956 3,253 14.20
4 CONCLUSIONS
The analysis revealed that different models of inter-
action must be included in the investigation of the ge-
netic background of biological phenomena, especially
in the case of studies with limited sample size. Ad-
ditionaly, looking at allelic frequencies and genotyp-
ing results only would limit the findings of the best
candidate biomarkers. The presented study demon-
strated that it is possible to design the proper statis-
tical analysis strategy even for small sample size de-
creasing significantly the false discovery rate for the
set of candidate SNPs. Due to limited power of statis-
tical tests applied, validation of candidate SNPs must
be performed by functional analysis and/or indepen-
dent validation experiment.
ACKNOWLEDGEMENTS
We would like to thank Dr. S. Majid, Ms. N.
Al-Harbi, Ms. S. Al-Qahtani for running the Ax-
iom Affymetrix platform and Sylwia Kabacik and
Paul Finnon for run the H2AX test. The work was
financially supported by NCN grant HARMONIA
UMO-2013/08/M/ST6/00924 (JP), the National In-
stitute for Health Research Centre for Research in
Public Health Protection at Public Health England
(CB), the National Science, Technology & Innova-
tion Plan (NSTIP) Project 11-BIO1429-20 (KFSHRC
RAC# 2120 003) (GA), and SUT grant BK/219/RAU-
1/2013/t.10 (JZ). Additionally, Joanna Zyla is holder
of scholarship DoktoRis - Scholarship program for In-
novative Silesia.
REFERENCES
Bergoni, J. and Tribondeau, L. (1906). De quelques rsultats
de la radiotherapie et essai de fixation d’une technique
rationnelle. Comptes-Rendus des Sances de l’Acadmie
des Sciences, 43:983–985.
Burnet, N., Elliott, R., Dunning, A., and West, C. (2006).
Radiosensitivity, radiogenomics and rapper. Clinical
Oncology, 18(7):525–528.
ModellingofGeneticInteractionsinGWASRevealsMoreComplex
RelationsbetweenGenotypeandPhenotype
207

Bush, W. and Moore, J. (2012). Chapter 11: Genome-wide
association studies. PLOS Comput Biol., 8(12).
Chung, S., Low, S., Zembutsu, H., Takahashi, A., ans
M. Sasa, M. K., and Nakamura, Y. (2013). A
genome-wide association study of chemotherapy-
induced alopecia in breast cancer patients. Breast
Cancer Res., 15(5):R81.
Finnon, P., Robertson, N., Dziwura, D., Raffy, C., Zhang,
W., Ainsbury, L., Kaprio, J., Badie, C., and Bouf-
fler, S. (2008). Evidence for significant heritability
of apoptotic and cell cycle responses to ionising radi-
ation. Hum Genet., 123(5):485–493.
Hindorff, L., Sethupathy, P., Junkins, H., Ramos, E., Mehta,
J., Collins, F., and Manolio, T. (2009). Potential etio-
logic and functional implications of genome-wide as-
sociation loci for human diseases and traits. PNAS,
106(23):9362–9367.
Kamboh, M., Demirci, F., Wang, X., Minster, R., Car-
rasquillo, M., Pankratz, V., Younkin, S., Saykin, A.,
Jun, G., Baldwin, C., Logue, M., Buros, J., Farrer,
L., Pericak-Vance, M., Haines, J., Sweet, R., Ganguli,
M., Feingold, E., Dekosky, S., Lopez, O., and Bar-
mada, M. (2012). Genome-wide association study of
alzheimer’s disease. Transl Psychiatry., 15:2:e117.
Klein, R., Zeiss, C., Chew, E., Tsai, J., Sackler, R., Haynes,
C., Henning, A., SanGiovanni, J., Mane, S., Mayne,
S., Bracken, M., Ferris, F., Ott, J., Barnstable, C.,
and Hoh, J. (2005). Complement factor h polymor-
phism in age-related macular degeneration. Science,
308(5720):385–389.
Lewis, C. (2002). Genetic association studies: design, anal-
ysis and interpretation. Brief Bioinform., 3(2):146–
153.
Lin, A., Wang, R., Ahn, S., Park, C., and Smith, D. (2005).
A genome-wide map of human genetic interactions in-
ferred from radiation hybrid genotypes. Genome Res,
20(8):1122–1132.
Lin, W. and Lee, W. (2012). Improving power of genome-
wide association studies with weighted false discov-
ery rate control and prioritized subset analysis. PLOS
One., 7(4).
NCI (2012). Radiation Therapy and You. National Institute
of Health publiaction No. 12-7157.
Niu, N., Qin, Y., Fridley, B., Hou, J., Kalari, K., Zhu, M.,
Wu, T., Jenkins, G., Batzler, A., and Wang, L. (2010).
Radiation pharmacogenomics: a genome-wide associ-
ation approach to identify radiation response biomark-
ers using human lymphoblastoid cell lines. Genome
Res, 20(11):1482–1492.
O’Donovan, M., Freemantle, M., Hull, G., Bell, D., Ar-
lett, C., and Cole, J. (1995). Extended-term cultures
of human t-lymphocytes: a practical alternative to pri-
mary human lymphocytes for use in genotoxicity test-
ing. Mutagenesis., 10(3):189–201.
Pahl, R. and Schafer, H. (2010). Permory: an ld-exploiting
permutation test algorithm for powerful genome-wide
association testing. Bioinformatics, 26(17):2093–
2100.
Ramensky, V., Bork, P., and Sunyaev, S. (2002). Human
nonsynonymous snps: server and survey. Nucleic
Acids Research, 30(17):3894–3900.
Roberts, S., Spreadborough, A., Bulman, B., Barber, J.,
Evans, D., and Scott, D. (1999). Heritability of cellu-
lar radiosensitivity: a marker of low-penetrance pre-
disposition genes in breast cancer? American Journal
of Human Genetics, 65(3):784–794.
Strachan, T. and Read, A. (1999). Human Molecular Ge-
netics. Wiley-Liss, New York, 2nd edition.
Taneja, N., Davis, M., Choy, J., Beckett, M., Singh, R.,
Kron, S., and Weichselbaum, R. (2004). Histone h2ax
phosphorylation as a predictor of radiosensitivity and
target for radiotherapy. J Biol Chem., 279(3):2273–
2280.
UNSCEAR (2010). Sources and effects of ionizing radia-
tion. United Nations Publication, New York.
Wrixon, A., Barraclough, I., Clark, M., Ford, J., Diesner-
Kuepfer, A., and Blann, B. (2004). Radiation, Peo-
ple and the Enviroment. International Atomic Energy
Agency, Austria.
BIOINFORMATICS2014-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
208
