
The Homologous Recombination Mathematical Model and the Role
of miRNAs in ATM/ATR-dependent and BRCA1/BRCA2-dependent
DSBs Repair Pathway
Outline of the Project
Katarzyna Jonak, Monika Kurpas and Krzysztof Puszyński
Systems Engineering Group, Institute of Automatic Control, Silesian University of Technology,
ul. Akademicka 16A, 44-100 Gliwice, Poland
1 INTRODUCTION
Eukaryotic cells are exposed continuously to the
genotoxic stresses caused by various sources, what
may result in formation of DNA double strand
breaks (DSBs) or single strand breaks (SSBs). DSBs
are known to be one of the most cytotoxic lesions,
caused by exposure to ionizing radiation (IR),
clastogenic drugs (Lindahl and Barnes, 2012), but
also formed endogenously during DNA replication
or even as an effect of reactive oxygen species
(ROS) (Lopez-Contreras and Fernandez-Capetillo,
2012). In order to maintain genomic integrity, the
DNA damage response is activated. This biological
signaling pathway is a cascade of the signals from
different types of macromolecules: detectors that
recognize DSBs, proteins mediating signal
transduction, and effectors responsible for activation
of damage response.
DSBs are detected indirectly by ataxia
telangiectasia mutated (ATM) that stabilizes and
activates repair pathways, such as homologous
recombination (HR) or non-homologous end joining
(NHEJ). The proper functioning of repair pathways
is essential to enhance the cellular survival.
For better understanding of the molecular
mechanisms of DNA repair pathways, the useful
approach is presented by systems biology. It
describes complex systems of interactions between
macromolecules with loops of positive and negative
feedbacks in a form of mathematical models. Such
modeling allows to not only understand the complex
interactions between various components of the
regulatory pathways, but also allows to investigate
the impact of DNA-damaging agents on cells that
can lead to such diseases as neurological disorders
or cancerogenesis. Mathematical models can be used
for preliminary analysis of the experimental
hypotheses, as well as for putting the hypotheses on
possible treatment at the level of the cellular
signaling pathways.
2 STAGE OF THE RESEARCH
2.1 DSBs Detector Module
ATM functions as a DSBs detector that sends the
signal about the damage to different mediators and
effectors. We have developed a mathematical model,
where the consequences of ATM activation on two
transcription factors, tumor antigen p53 and NF-κB
(nuclear factor kappa-light-chain-enhancer of
activated B cells), are presented. Both nuclear
factors control several physiological processes from
cell cycle arrest through DNA repair and adaptive
immune response to apoptosis. The model is based
on our previous model of p53-NF-κB interaction
(Puszynski et al., 2009).
Major DNA damage response regulators play an
essential role in ATM-p53-NF-κB pathway. Mdm2
(E3 ubiquitin-protein ligase) facilitates p53
degradation, checkpoint kinase 2 (Chk2) inhibits p53
ubiquitination and degradation, and cellular
transcription factor CREB transcriptionally activates
ATM. Moreover, in this model we linked ATM-p53-
NF-κB pathway components with protein
phosphatase Wip1 that regulates dephosphorylation
events (inactivation of the most of the pathway
components).
The mathematical model is presented as a set of
stochastic and deterministic equations according to
the Haseltine-Rawlings postulate. Stochastic
description and following Gillespie direct method
based simulation were used for slow reactions, like
states of genes change, while deterministic
41
Jonak K., Kurpas M. and Puszy
´
nski K..
The Homologous Recombination Mathematical Model and the Role of miRNAs in ATM/ATR-dependent and BRCA1/BRCA2-dependent DSBs Repair
Pathway - Outline of the Project.
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
description based on ordinary differential equations
(ODE) and following Runge-Kutta 4
th
order
simulation method were used for description of
quick reactions, like activation or degradation of the
proteins involved in repair pathway.
The model is activated upon IR induction and
TNFα (tumor necrosis factor alpha). The signaling
pathway is also stimulated continuously by the small
number of damages that occur spontaneously. The
components of ATM-p53-NF-κB model are
presented mostly in two main states: active and
inactive. Moreover, most of the proteins considered
in the model contain their transcriptional forms
(mRNA). The model has an assumption that each
gene has two copies and among them one can be in
an active state, both, or none.
The output of the model is p53 level that
determinates cell fate. In this model cell death is
recognized as a permanently increase of p53 level
for more than 6 hours, then cell is considered as an
apoptotic and its elements are degraded.
We simulated the cellular response to the
damage combining all of the described elements.
The obtained results shown that ATM pathway is an
effective system for DSBs detection with strong
amplification signal and quick response.
Furthermore, we observed the strong dependence of
the cellular response to the DNA damage on Wip1,
what leads to the conclusion that it plays a role as
a gatekeeper in the ATM-Mdm2-p53 regulatory
loops, essential in the process of DNA damage
repair.
2.2 SSBs Detector Module
Another detector system which was important in our
work was ATR module (ataxia telangiectasia and
Rad3-related protein) responsible for detection of
DNA SSBs caused by, for example, ultraviolet
radiation (UV) or replication fork arrest (Lopez-
Contreras and Fernandez-Capetillo, 2012). The main
subject of the study was to develop a mathematical
model for p53 regulatory pathway with ATR as
a main detector system, perform simulations and
then linked to NF-κB regulatory module, ATM
detection module and deactivation agent Wip1.
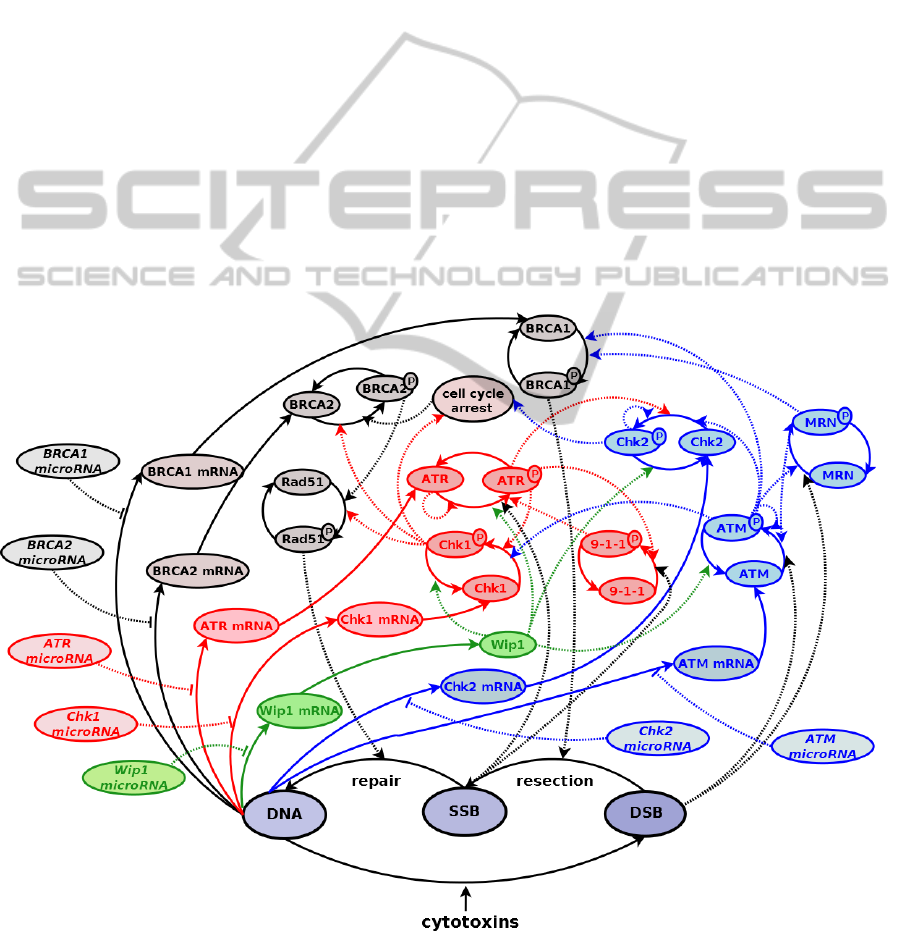
Figure 1: Schematic model of HR repair pathway with ATM and ATR as major detector systems of DSBs and SSBs. The
letter “P” next to the protein name indicates phosphorylated form of this protein. Solid lines are transitions between states of
the HR components, dotted lines with arrow-heads are positive regulation (acceleration), and dotted lines with hammer-
heads are negative regulations (inhibition).
BIOSTEC2014-DoctoralConsortium
42

In ATR-p53 model the first stage of SSBs detection
is an activation of Rad9-Rad1-Hus1 protein complex
(called 9-1-1) and activation of the ATR-ATRIP
(ATR interacting protein) complex. Rad9 subunit
phosphorylated by protein ATR recruits TopBP1
(topoisomerase II binding protein 1), which presence
is necessary for full activation of ATR. Then, ATR
activates checkpoint kinase 1 (Chk1) that activates
p53 and increase degradation rate of Mdm2.
The model was built using the same assumptions
as ATM model, with the usage of stochastic and
deterministic approaches. The activation of the
ATR-p53 model was performed upon UV radiation
at 24 hours after start of the simulations. The effect
of different doses of UVC was studied and the
apoptotic death threshold was chosen. It was
observed in situ that with dose of 18 J/m
2
more than
half of the cells become apoptotic.
The simulations shown that ATR module acts as
an efficient system to detect even a single DNA
damage. The SSBs detection module is very fast -
the breakage is detected within few seconds after the
occurrence of DNA damage. Moreover, ATR and
ATM mathematical models explain that the base
production and activation level of the p53 protein
and its signaling pathway proteins may be caused by
persistent cellular stress.
2.3 HR as a Repair Pathway
The interesting subject in the area of modeling DSBs
and SSBs detection modules is the role of the
different proteins responsible for specific repair
pathways. Therefore, now we combine the two
detector modules, ATM and ATR, in order to
perform further extensions to homologous
recombination repair pathway.
We have already investigated the main modules
responsible for HR. The main interactions between
them are presented in Figure 1. The detector system
of DSBs is ATM, which together with MRN
complex activates BRCA1 (breast cancer type 1
susceptibility protein) responsible for DNA repair.
This activation leads to resection of DSBs and
activation of ATR module that detects SSBs. Then,
the process of repairing the single strand damages
starts and goes through BRCA2 (breast cancer type
2 susceptibility protein) and Rad51. The model
includes the effects of the damages on checkpoints
proteins, as well as the effect of known specific
microRNAs (miRNAs) responsible for regulation of
transcription of the proteins involved in HR
pathway.
3 OUTLINE OF OBJECTIVES
For a better understanding of the HR pathway and
identification of the abnormalities that may occur in
this process, it is useful to build a mathematical
model describing the dynamics of this module. The
model should be based on experimental data in order
to correspond to reality, hence the biological model
will be built that will allow experimental verification
of the HR mathematical model.
The main goal of the project is to examine the
impact of different agents that cause DSBs on HR in
a single cell as one of the most important repair
pathways in eukaryotic organisms. For this purpose
the models of ATM and ATR pathways will be
combined as detectors active in S/G2 phase of cell
cycle without taking into account interactions with
p53 and NF-κB. The whole HR pathway will be
described, from detector module to ligation of the
DNA strands. Another objective is to identify
substances which can cause DSBs without causing
SSBs. The impact of the DNA damages on miRNAs
will be also investigated with the negative
interaction of these macromolecules on other
components of HR pathway.
Within the project we anticipated three tasks:
Construction of the deterministic and
stochastic mathematical model of HR repair
pathway based on the information from
existing models described in the literature, as
well as ATM and ATR pathways models
developed by the authors.
Collection of the data from experiments and
literature on the parameters of the model, such
as activation and inactivation rates,
degradation rates, transcription rates, etc.
Construction and experimental verification of
the model.
4 RESEARCH PROBLEM
The main research problem focuses on the
interactions between different components of HR
pathway during repair process of DNA double strand
breaks. Because of the fact that HR occurs only in
S/G2 phase, some of the proteins, such as p53, are
not active, what should be taken into account during
the model development. It is necessary to note that
different concentrations of specific detection
components, such as MRN, may lead to various
repair pathways, for example, NHEJ. This
TheHomologousRecombinationMathematicalModelandtheRoleofmiRNAsinATM/ATR-dependentand
BRCA1/BRCA2-dependentDSBsRepairPathway-OutlineoftheProject
43
phenomena should be also considered in the
theoretical model.
The impact of miRNAs on HR components is
essential during repair. Overexpression of some of
the miRNAs may cause cell cycle arrest and may
forward the cell into apoptosis pathway through p53
protein in G1 phase. The same result may be
obtained by downregulation of BRCA1 or BRCA2
genes and low activation level of these proteins.
Therefore, the exact impact of these components
should be examined, as well as the impact of Wip1 –
the major deactivation agent.
We found necessary to examine which DNA-
damaging factors may lead to DSBs and to
activation of HR pathway, as well as what dose of
these factors may lead to cell cycle arrest and
apoptosis.
The main purpose of the existence of the HR
model with the DNA damages detector modules is to
illustrate the processes occurring during DNA repair.
The project will allow to understand the interactions
between molecules, probably investigate new
components of the HR network, and contribute to
putting the hypotheses on possible treatment of
different diseases at the level of the cellular
pathways.
5 STATE OF THE ART
5.1 DSBs Detection
Activation of a specific mechanism of DNA repair
depends mainly on cell cycle. Thus, initiation of
NHEJ is possible during the whole cycle, however
usually appears in G1/G0 phases, where HR is
limited to late S phase and G2 phase (Langerak and
Russell, 2011). DSBs are detected by ATM and
multiprotein complex MRN. In G1 phase, the
activation of these proteins results in Chk2-mediated
p53-dependent cell cycle arrest. DNA damages
undergo only minor nucleolytic processing being
repaired very fast by NHEJ (Jazayeri et al., 2006). In
case of S/G2 phases and repair by HR, the cell cycle
arrest is p53-independent and the process of repair is
slower than NHEJ (Jazayeri et al., 2006). The choice
between HR and NHEJ repair pathways is partially
determinate by MRN complex activity: in HR when
DSBs require resection the activity of the complex is
much higher (Chowdhury et al., 2013). BRCA1 also
promotes resection and excludes 53BP1 protein,
which is involved in NHEJ process (Chowdhury et
al., 2013).
ATM is activated by DSBs directly and
indirectly by MRN complex. ATM is also
autophosphorylated by the formation of defects in
the chromatin structure (Bakkenist and Kastan,
2003). At the same time exonulease MRN is
activated directly by DSBs and by phosphorylation
of one of its component, Nbs1, by ATM (Bakkenist
and Kastan, 2003). ATM is involved in cell cycle
arrest by phosphorylation of Chk1 and mostly by
phosphorylation of Chk2. The signal of DSBs is
amplified by autophosphorylation of Chk2 induced
by ATM (Ahn et al., 2004).
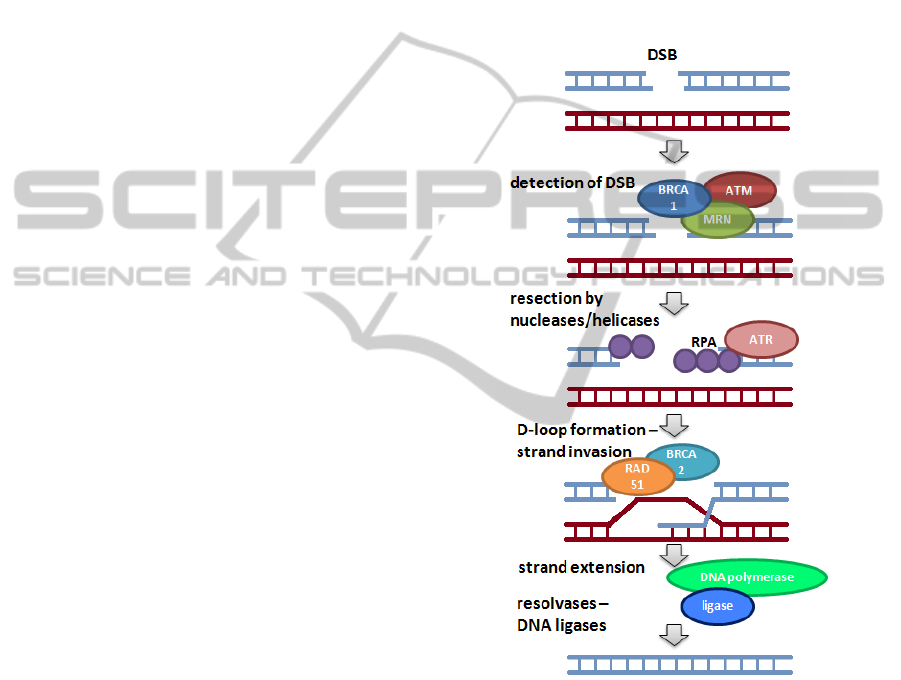
Figure 2: Scheme of HR repair pathway. DNA helix after
DSBs induction is presented as a light blue ladder, DNA
from sister chromatid is a purple ladder.
5.2 DNA Resection and Repair
MRN together with CtIP protein binds to free ends
of DNA molecule and in presence of BRCA1
degrades one of the two helices from 5' end of DNA
strand. This process results is resection of DSBs.
The resulting free 3' ends are detected and protected
by replication protein A complex (RPA) (Filippo et
al., 2008). These damages of single stranded DNA
(ssDNA) recruits proteins involved in detection of
BIOSTEC2014-DoctoralConsortium
44

SSBs, such as ATR. ATRIP is capable of attaching
themselves to the RPA-ssDNA, which induces
autophosphorylation of ATR (Nam et al., 2011). In
addition, ssDNA fragment binds a complex of
Rad17-RFC2-5, which allows the attachment of the
9-1-1 complex to the damaged site (Filippo et al.,
2008). ATR recruits TopBP1 and then activates
several proteins involved in the repair pathway, such
as Chk1 and Chk2. Signal strength of the checkpoint
cascade is dependent on the length of RPA-ssDNA
and the possibility of autophosphorylation of ATR
molecules (Filippo et al., 2008).
Activated Chk1 and Chk2 results in cell cycle
arrest by inactivation of cyclin-dependent kinases.
The reduction of activity of these kinases leads to
activation of BRCA2, which recruits Rad51 and
interact with RPA-ssDNA to initiate HR process
(Filippo et al., 2008). Moreover, activated Chk1
phosphorylates Rad51, what leads the cell to the HR.
BRCA2 and RAD51 together with the
accompanying proteins catalyze the invasion of the
free 3' end to the homologous sequence of sister
chromatid. As an effect the D-loop structure is
formed, what allows for the rebuilding of a missing
DNA fragment by polymerase η (Filippo et al.,
2008). The DNA ligase I join the new fragments.
Synthesis is finished when two Holliday’s structures
are formed (Filippo et al., 2008). These structures
are then removed by helicases, like BLM,
topoisomerases IIIα or endonucleases, such as
Mus81/Eme1 (Filippo et al., 2008). The process of
DNA repair by HR is presented in simplified way in
Figure 2.
5.3 MiRNAs and Wip1 in HR Pathway
An essential component in regulation of DSBs
detection and the process of DNA damages repair is
Wip1, which is a deactivation agent for key proteins
involved in HR. The main role of this protein is to
regulate the level of activated proteins mostly after
succeed process of repair, and to unlock the cell
cycle. Wip1 transcription is p53-dependend, what
makes it connected to DNA damages (Lowe et al.,
2012). The most important parts of regulation of the
repair pathways by Wip1 is associated with
inactivation of DNA damages detector modules:
ATM and ATR. Moreover, the cell cycle
checkpoints, Chk1 and Chk2, are dephosphorylated
by Wip1, as well as p53 and Mdm2 (Lowe et al.,
2012).
Small RNAs, called miRNAs, also play
important roles in DSBs repair pathway. Both
miRNA transcription and maturation processes are
altered in response to damages of DNA strands and
repair processes. Biogenesis of these
micromolecules is induced in an ATM-dependent
manner (Chen X. and Chen T., 2011). Activated
ATM phosphorylates KSRP protein (KH-type
splicing regulatory protein) what leads to activation
of some of the miRNAs (Chen X. and Chen T.,
2011). Not all of the miRNAs involved in HR
pathway have been discovered yet, however there
are some which are known, such as miR-100, miR-
101 and miR-421 which suppress ATM, miR-182
which suppress BRCA1 or miR-16 that suppress
Wip1 (Chen X. and Chen T., 2011).
5.4 Existing HR Models
The existing models of HR pathway are based
mainly on the late phase of the repair process: from
RPA coating to the action of DNA ligases.
In the model described in (Taleei et al., 2011)
DSBs detection module is treated as a one
component: MRN. The authors focus on the effects
of resection, without describing the whole DSBs
detection with ATM, and without including
checkpoint proteins. The most important parts there
are MRN, RPA, Rad52 and ligase. Another
theoretical model (Rodríguez et al., 2012) is based
on Boolean network system for the FA/BRCA
pathway involved in HR. The whole model is very
expanded and it takes into account also NHEJ repair
pathway. However, the model do not contain the
effects of miRNAs and is rather focus only on the
whole BRCA pathway.
6 METHODOLOGY
The mathematical model of HR repair pathway will
be built using set of equations that allow simulating
the behavior of one cell treated with different doses
of cytotoxins that cause DSBs. For the mathematical
model stochastic and deterministic approach will be
used as it was performed for the models of ATM and
ATR signaling pathways. Moreover, the Michaelis-
Menten kinetics and the law of mass action will be
used in order to bring the model and its reaction
speed to reality.
In order to investigate the effect of other not-yet-
known modules on described components of the HR
pathway, several biological experiments will be
performed. Northern blotting will be used in order to
investigate miRNAs that regulate different
components of the HR. Western blotting will be
used to investigate level of key proteins (total and
TheHomologousRecombinationMathematicalModelandtheRoleofmiRNAsinATM/ATR-dependentand
BRCA1/BRCA2-dependentDSBsRepairPathway-OutlineoftheProject
45

phosphorylated forms) involved in the process of
DSBs detection and repair, as well as to collect the
information concerning the kinetics parameters of
the model, such as time of deactivation of the
specific protein. Moreover, the analysis of the
expression profile of genes involved in HR pathway
will be performed with a method of quantitative
real-time PCR in stress conditions.
The analysis of the level of double strand breaks
of DNA after treatment with specific cytotoxins will
be performed with microscope analysis of H2AX
foci assay. The amount of foci reflects the amount of
DNA breaks. The analysis of the total number of
DSBs and SSBs will be performed with comet assay.
The experiments will be performed on
mammalian cancer and normal cell lines with active
or inactive forms of the proteins involved in HR.
The whole methodology is still under development.
7 EXPECTED OUTCOME
The experimental-based mathematical model
(stochastic and deterministic) of HR repair pathway
will be presented. The HR model, together with
ATM and ATR models developed by our group, will
create a comprehensive mathematical model
describing the dynamics of the interactions
occurring in the cell from the inception of DNA
damages to making the decisions about cell fate: to
direct cell to repair by HR, to arrest cell cycle or
direct cell to apoptosis pathway during HR or before
the process of repair.
The expected outcome is an identification of at
least one new component of the HR pathway which
can be even protein or miRNA, and the parameters
of the model for most of the pathway components.
Moreover, it is expected that the developed model
will reflect the experimental data and will be a good
tool for simulation of the cell behavior during HR.
REFERENCES
Ahn, J., Urist, M., Prives, C., 2004. The Chk2 protein
kinase. DNA Repair, 3:1039-1047.
Bakkenist, C. H., Kastan, M. B., 2003. DNA damage
activates ATM through intermolecular
autophosphorylation and dimer dissociation. Nature,
421:499-506.
Chen, X., Chen, T., 2011. DNA repair, InTech. 1
st
edition.
Chowdhury, D., Choi, Y. E., Brault, M. E., 2013. Charity
begins at home: non-coding RNA functions in DNA
repair. Nature Reviews Molecular Cell Biology,
14:181-189.
Jazayeri, A., Falck, J., Lukas, C., Bartek, J., Smith,
G.C.M., Lukas, J., Jackson, S.P., 2006. ATM- and cell
cycle-dependent regulation of ATR in response to
DNA double-strand breaks. Nature Cell Biology, 8:37-
45.
Langerak, P., Russell, P., 2011. Regulatory networks
integrating cell cycle control with DNA damage
checkpoints and double-strand break repair.
Philosophical Transactions of the Royal Society,
366:3562-3571.
Lindahl, T., Barnes, D. E., 2012. Repair of endogenous
DNA damage. Cold Spring Harb Symp Quant Biol,
65:127-3.
Lopez-Contreras, A. J., Fernandez-Capetillo, O., 2012.
Protein Phosphorylation in Human Health, InTech. 1
st
edition.
Lowe, J., Cha, H., Lee, M. O., Mazur, S. J., Appella, E.,
Fornace, A. J., (2012). Regulation of the Wip1
phosphatase and its effects on the stress response.
Frontiers in Bioscience, 17: 1480-1498.
Nam, E. A., Zhao, R., Glick, G. G., Bansbach, C. E.,
Friedman, D. B., Cortez, D., 2011. Thr-1989
Phosphorylation Is a Marker of Active Ataxia
Telangiectasia-mutated and Rad3-related (ATR)
Kinase. J Biol Chem, 286(33): 28707–28714.
Puszynski, K., Bertolusso, R., Lipniacki, T., 2009.
Crosstalk between p53 and nuclear factor-kB systems:
pro- and anti-apoptotic functions of NF-kB. IET Syst
Biol., 3(5):356-367.
Rodríguez, A., Sosa, D., Torres, L., Molina, B., Frías, S.,
Mendoza, L., 2012. A Boolean network model of the
FA/BRCA pathway. Bioinformatics, 28(6):858-66.
San Filippo, J., Sung, P., Klein, H., 2008. Mechanism of
Eukaryotic Homologous Recombination. Annu. Rev.
Biochem,. 77:229–57.
Taleei, R., Weinfeld, M., Nikjoo, H., 2011. A kinetic
model of single-strand annealing for the repair of
DNA double-strand breaks. Radiat Prot Dosimetry.,
143(2-4):191-5.
BIOSTEC2014-DoctoralConsortium
46
