
Wize Sniffer
A New Portable Device Designed for Selective Olfaction
Mario D’Acunto
1,2
, Antonio Benassi
1
, Federica Chiellini
3
, Danila Germanese
1,4
, Randa Ishak
5
,
Massimo Magrini
1
, Emanuele Pagliei
6
, Paolo Paradisi
1
, Marco Righi
1
and Ovidio Salvetti
1,2
1
Istituto di Scienza e Tecnologie dell’Informazione ISTI-CNR, via Moruzzi 1, 56124 Pisa, Italy
2
NanoICT Laboratory, Area della Ricerca CNR di Pisa, via Moruzzi 1, Pisa, Italy
3
Department of Chemistry and Industrial Chemistry, University of Pisa, Pisa, Italy
4
Department of Information Engineering, University of Pisa, Pisa, Italy
5
Department of Civil and Industrial Engineering, University of Pisa, Pisa, Italy
6
Cosmed Srl, via dei Piani di Monte Savello 37, 0041 Pavona di Albano, Rome, Italy
Keywords: Breath Analysis, Portable Device, Statistical Data Processing, Chemical Sensors.
Abstract: Digital semeiotics is one of the newest recent challenges for assessing a number of computational
descriptors to atherosclerotic cardiovascular diseases that are leading causes of mortality worldwide. These
descriptors can be expressed involving (i) morphometric, biometrics and colorimetric of the face; (ii)
spectroscopic analysis of skin and iris, of sub-cutaneous substances and the function of subcutaneous
tissues, and (iii) compositional analysis of breath and exhaled. In this paper, we describe the design and
functionality of the Wize Sniffer (WS), a new portable device for breath analysis limited to an effective
number of substances. Within the SEMEOTICON Project by the WS, we intend a hardware/software tool
for both the analysis of volatile organic compounds of breath and a platform for data mining and data
integration. The WS should be able to provide useful information about the “breathprint”, i.e., the analog of
fingerprint for the state of health of an individual.
1 INTRODUCTION
Atherosclerotic cardiovascular diseases (ACDs)
represent the leading cause of worldwide mortality
(World Health Organization. The Global Burden of
Disease: 2004 and update 2008; (Lison et al., 2010)).
Breath gases are recognized to be excellent
indicators of the presence of diseases and clinical
conditions. Such gases have been identified as
biomarkers using instrumentations such as gas
chromatography (GC) or electronic nose (e-nose)
(Guo et al., 2007). GC is very accurate but
expensive, time consuming and non portable. E-nose
has the advantages of low-cost and easy operation,
but is not particularly useful for analyzing breath
substances. As a consequence, in recent years, the
necessity to develop a portable device for breath
analysis, easy to use, and feasible for patients living
far from medical structures or physicians.
Essentially, a design of a portable device for
breath analysis is based on selected chemical sensors
that are sensitive to the biomarkers and compunds in
breath substances and make use of accurate
statistical tools for odor signal preprocessing,
classification methods and, in turn, identification of
possible diseases if any. In SEMEOTICONS
activity, we are developing a device following the
above requirement. The device, called Wize Sniffer
(WS), captures breath samples, the chemical
selective sensors sense the sample and accordingly
form a sort of odorprint of healthy people or patients
with known and specific diseases, in order to
evaluate the well-being state of a human subject
(Miekish et al., 2004). It should be noted that does
not exist a general definition of well-being, rather
some indices for well-being that can be correlated to
cardio-metabolic risk. In addition, such indices are
strictly connected to the recognition of behavioural
trends and the methods for computing the raw
numerical values of such well-being indices must be
designed for single individual. However, one critical
index of well-being is given by the breath
composition. Human breath is largely composed of
oxygen, carbon dioxide, water vapour, nitric oxide,
577
D’Acunto M., Benassi A., Chiellini F., Germanese D., Ishak R., Magrini M., Pagliei E., Paradisi P., Righi M. and Salvetti O..
Wize Sniffer - A New Portable Device Designed for Selective Olfaction.
DOI: 10.5220/0004938605770582
In Proceedings of the International Conference on Health Informatics (SUPERHEAL-2014), pages 577-582
ISBN: 978-989-758-010-9
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

and numerous volatile organic compounds (VOCs)
(Di Francesco et al., 2005); (D’Amico et al., 2007).
The type and number of VOCs in the breath changes
among different individuals, but there is a common
core of breath VOCs which are present in all
individuals. The molecules in an individual’s breath
may be exogenous or endogenous. Exogenous
molecules are those that have inhaled or ingested
from the environment or other sources such as air or
food, thus giving no diagnostic value. Endogenous
molecules are produced by metabolic processes and
partition from blood via the alveolar pulmonary
membrane into the alveolar air. Such endogenous
molecules are present in breath relatively to their
types, concentrations, volatilities, lipid solubility and
rates of diffusion as they circulate in the blood and
cross the alveolar membrane. Changes in the
concentration of the molecules in VOCs could
suggest various diseases or at least changes in the
metabolism.
In this paper, for the first time we present the
design of a portable device able to operate with a
limited number of breath VOCs, normally connected
to oxidative stress, thus giving information to
physicians on the possible state of wellness of an
individual.
2 BREATH ANALYSIS AND
WELLNESS STATE
In this section, we describe the correlation between
exhaled VOCs and oxidative stress. Oxidative stress
is the cause of over 100 diseases, including
atherosclerosis or hypertension. In addition,
hypertension is a risk factor that can lead to
myocardial injury, cardiac failure, aortic aneurysm.
The design of a portable device for breath analysis
must necessarily work on a limited space of breath
VOCs. The most relevant VOCs and their
connection to oxidative stress are labelled as
follows.
Carbon Monoxide (CO): it is naturally produced
by the action of heme oxygenase on the heme for
haemoglobin breakdown. This produces
carboxyhemoglobin, which is a more stable
molecule than oxyhemoglobin. For example, an
increase of CO leads haemoglobin to carry less
oxygen through the vessels. CO is present in
cigarette smoke, and air pollution too. In addition, it
is a blood vessel relaxant (vasodilator), and a
promoter of neurovascular growth, therefore it could
be considered as an indicator of brain tumor growth.
Carbon Dioxide (CO
2
): the variation rate of CO
2
can be associated to different metabolic response,
for example, CO
2
increases during physical activity
or in general with the increment of blood-heat as in a
fever. Analogously, there is a decrease in the case of
hypothermia. In healthy individuals, partial pressure
in arterial blood is very close to the partial pressure
in expired gases. In most forms of lung diseases and
some of congenital heart disease (cyanotic lesions-
bluish-grey discoloration of the skin, lack of O
2
in
the body), a decrease of CO
2
exhaled rate is
commonly observed. Capnography is a technique
commonly used for monitoring the concentration of
CO
2
, or, similarly, its partial pressure using such
technique, the max value of a capnogram
corresponds to the end of tidal volume of exhaled
breath and the steady-state concentrations of each
breath. It must be noted that the breathing rate
influences the level of CO
2
in the blood: slow
breathing rates cause Respiratory Acidosis (i.e.,
increase of blood CO
2
partial pressure, that may
stimulate hypertension, or heart rate acceleration).
On the contrary, too rapid breathing rate leads to
hyperventilation, which may provoke Respiratory
Alkalosis (i.e., decrease of blood CO
2
partial
pressure, no longer fits its role of vasodilator,
leading to possible arrhythmia or heart trouble).
Ethanol: the ethanol compound in a breath
composition can be classified as endogenous or
exogenous. Ethanol endogenous may increase in
exhaled gas mixtures because of alcoholic
fermentation of an excessive over-load of
carbohydrates. Indeed it is originated from microbial
fermentation of carbohydrates in the gastro-intestinal
tract. Then, it enters in the blood. On the contrary,
Ethanol exogenous comes from alcoholic drinks. It
is important to note that it is recognized that ethanol
breakdown leads to an accumulation of free radicals
into the cells, a clear example of oxidative stress.
Ethanol may cause arrhythmias and depresses the
contractility of cardiac muscle.
Nitrogen Oxide (NO): it is a vasodilator and it
modulates inflammatory response (operating in
combination with CO and Hydrogen Sulfide).
Hydrogen Sulfide (H
2
S): it is a vascular relaxant
agent, and has a therapeutic effect in various
cardiovascular diseases (myocardial injury,
hypertension). In general, H
2
S could have
therapeutic effect against oxidative stress due to its
capability to neutralize the action of free radicals. In
patients with coronary heart disease, H
2
S level in
blood is normally reduced, as in the case of
hypertension. On the contrary, in haemorrhagic
shock, H
2
S plasma levels are increased.
Ammonia (NH
3
): an increase of NH
3
in blood
HEALTHINF2014-InternationalConferenceonHealthInformatics
578

may be caused by cigarette smoke, renal failure,
cardiac failure, changes in cardio-circulatory system.
Hydrogen and Methane: they are produced by
the breakdown of the carbohydrates in the colon by
anaerobic bacteria. They can be correlated to
overweight problems, and probably to an improper
life-style. Nevertheless, their quantity is very low
presenting some problems for their detection.
Cigarette smoke: it contains principally Nicotine,
CO, NH
3
, oxidant substances leading to oxidative
stress. After 1 minute smoking, an increase of heart
rate can be revealed. Tobacco combustion causes a
constriction of blood vessels.
Other factors leading to oxidative stress are
cholesterol and fibrinogen. An increase of
cholesterol leads to atherosclerotic issues and,
similarly, an increase of fibrinogen leads to blood
viscosity. In addition, a strong correlation exists
between exhaled hydrocarbons and cardiovascular
diseases, principally pentane and ethane. In healthy
subjects, there is a balance between oxidant
molecules (free radicals) and anti-oxidant molecules.
When free radicals are balanced, they have a
positive effect on the general person wellness and
health, because they are involved in many
inflammatory processes, thus having a therapeutic
potential. On the contrary, when free radicals
number exceeds the anti-oxidant ones, this leads to
oxidative stress.
3 THE WS DESIGN
The design of the WS platform device operates in
three phases: gas collection, sampling and data
analysis. During the first phase the subject breaths
into a gas sampling box. Our design follows the
recent proposal by Guo et al., (2007). The gas is
injected into the chamber containing a sensor array.
Then, a measurement circuit measures the
interaction between the breath and the array sensors.
Then signals are filtered and amplified and
afterwards sent to computer for further analysis. The
WS components are array sensors contained in a
customized box, the microcontroller board and the
statistical analysis tool.
The array sensors are both commercial and
customized with conductive polymers electrospun
nanofibers as sensing material. When using a
conductive polymer, it is reasonable to have an
increased sensor sensitivity and, moreover, the
possibility to measure nicotine concentration. Note
that a correspondent commercial sensor for nicotine
is not available.
Table 1: Specific VOC and corresponding sensitive gas
capability of Figaro sensors.
Breath substance
Baseline levels
(ppm)
Sensors
Sensitivities (ppm)
CO
2
(in percentage
4%)
40000 30-300
CO
3.5 (14-30 with
moke)
50-1000
Ethanol
0.62
153-180
1-10
50-1000
Hydrogen sulfide 0.33 0.1-1
Ammonia 0.42-0.46
1-10
30-300
Hydrogen 9.1
1-10
10-100
The used commercial chemical sensors are
provided by the Figaro Engineering Inc. These
sensors are very sensitive, robust and resistant to
humidity and ageing. In table 1, the sensitivities of
such sensors for any VOC to be detected are
reported.
Any chemical sensor should have has unique
odour-print corresponding to the capacity to sense a
single VOC substance. The framework of the WS
consists of three modules: signal measurement,
signal conditioning and signal acquisition. The array
of sensors is contained in the signal measurement
module. The measurement circuit is responsible for
transforming gas signals into electronic signals. The
array sensors are mounted directly or connected with
a griddle to a microcontroller board that can operate
on the data collected filtering and amplifying them.
The microcontroller used in our system is a widely
employed open source controller: Arduino 2560
system that can be powered very simply via a USB
connection (see Figure 1).
Figure 1: The microcontroller used for the electronic data
collection: Arduino Mega 2560 system (based on the AT
mega 2560), that can be powered very simply via a USB
connection. The VOCs data collected can be loaded on
Arduino or sent to computer with the USB connection.
The length of the Mega 2560 system is 10 cm.
WizeSniffer-ANewPortableDeviceDesignedforSelectiveOlfaction
579
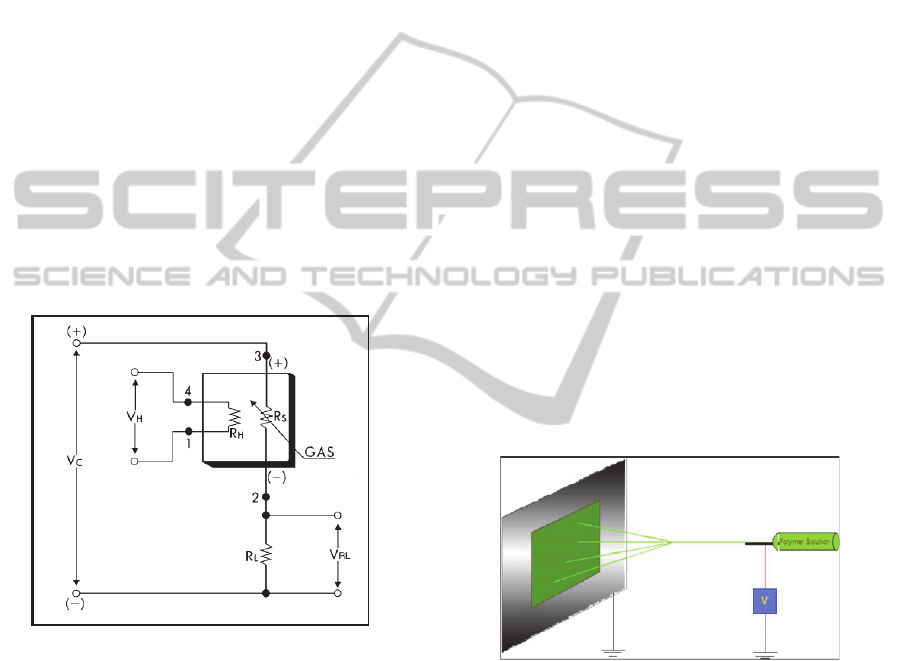
Figaro Engineering provides the chemical
sensors and a general purpose electric scheme to
adopt according to the specific use. The ability to
sense a selective VOC substance depends on the
employed sensing materials. For example,
considering the sensor named TGS2600, able to
sense CO, Ethanol and iso-butane, the
manufacturing procedure is as follows: using thick
film techniques, the sensor material is printed on
electrodes (noble metal) that have been printed onto
an alumina substrate. The main sensing material of
the sensor element is tin dioxide (SnO2). One
electrode of the sensing material is connected to a
pin 2 (see Figure 2) and the other is connected to
another pin 3. An RuO2 heater printed onto the
reverse side of the substrate and connected to pins 1
and 4 heats the sensing material. Lead wires are Pt-
W and connected to sensor pins which are made of
Ni-plated Ni-Fe 50%. The sensor base is made of
Ni-plated steel. The sensor cap is made of stainless
steel and contains 6 pin holes on the top of the
sensor. The basic measuring circuit is represented in
Figure 2.
Figure 2: Basic measuring circuit of Figaro chemical
sensors.
Circuit voltage (VC) is applied to the electrodes of
the sensor element. The behaviour of the sensor
element is that of a variable resistance (RS). In order
to accord the circuit to the acquiring device it is
necessary to use the load resistor (RL). Vc is always
required for the circuit power supply, and the
polarity shown in Figure 2 must be maintained. The
sensor value is measured indirectly as a change in
voltage across RL and RS. The
signal measurement module measures these voltages
and converts them into digital signals. The analog
signals are then conditioned by signal filtering and
amplifying. The sampling procedure is based on four
steps described as follows:
1) before the sampling, the chamber is purged and
the sensor returns to a steady state. The baseline
value is measured and recorded for data
manipulation and normalization;
2) 0~5s (injection stage): sampled gas is injected
into the chamber at an invariable rate. The
particles of sampled gas that are injected and
accrue inside the chamber produce a resistance
change and cause the amplitude of the signal to
rise;
3) 6 ~10s (reaction stage): particles in the chamber
continue to accumulate on the sensors but the
accumulation rate is decreasing. The resistance
of the sensor monotonically increases at a
decreasing rate, as the amplitude of the signals;
4) 15~90s (purge stage): the chamber is purged
again. The pump quickly draws out the
remaining analyte, thereby shortening the
sampling time as well as refreshing the air for the
next use.
3.1 Electrospun Nanofibers as Sensing
Materials
The sensitivity of commercial sensors, as well as
selectivity, can be improved reducing the sensing
area. This achievement can be obtained using as
sensing material conductive polymers elestrospun
nanofibers, (see Figure 3).
Figure 3: A schematic sketch of electrospinning process.
Electrospinning is a manufacturing process able
to produce ultra-fine fibers with diameters ranging
from 50-500nm (Ding et al., 2009); (Chen et al.,
2011). In the electrospinning process an electric
field is generated between a polymer solution and a
metal collection screen (collector). The polymer
solution is contained in a syringe with a steel
capillary tip and a voltage is applied between the tip
and the conductive collector. As the electrical
potential is increased, the charged polymer solution
is attracted to the collector. When the voltage
reaches a critical value, the charge overcomes the
surface tension of the polymer solution formed on
HEALTHINF2014-InternationalConferenceonHealthInformatics
580

the capillary tip and an electrified jet is produced. As
the charged jet flies through the air, it loses the
solvent through a quick evaporation and impinges on
the collection screen as a thin fiber mat, as shown in
Figure 4.
Figure 4: Example of electrospun nanofibers as observed
at a Scanning Electron Microscope.
In the development of the WS, we will use
electrospun nanofibers as sensing material and
conductive polymers, such as polyaniline (PANi),
polypirrole (PPY), poly(p-phenylene vinylene)
(PPV) and others to be defined yet (Slater et al.,
1992); (Slater et al., 1993); (Savage et al., 2001).
The advantage of sensors using nanofibers as
sensing material would be a general increment of
sensitivity and a significant reduction of the
response time. Nevertheless, such properties must be
evaluated during the measurement stages, and the
possible response of such sensors is beyond the
scope of the present paper.
3.2 Statistical data processing
Since the operability of WS works on a reduced
space of breath substances, data analysis requires a
particular care because of the various sources of
errors. The sensors measure changes in voltage
across each sensor and convert the raw signal into a
digital value that can be managed by physicians. Our
analysis of sensor data requires three steps: signal
preprocessing, feature extraction and classification.
The aim of signal preprocessing is to compensate
drifts and to eliminate irrelevant information so to
improve the performance of the subsequent pattern
recognition and classification. It is assumed that the
dynamic response of any sensors is sampled
introducing reasonable time intervals in a particular
way for the transient phase. In addition,
normalization is used to compensate for sample-to-
sample variations caused by analyte concentrations
and pressure of oxygen. At this point, we introduce
feature extraction from any sample using the
standard procedure of principal component analysis
(PCA) and, finally, k-nearest neighbor voting rule
(KNN) will be used as a classifier for the features
that extracted by PCA, (Guo et al., 2007).
4 CONCLUSIONS
In this paper, we have presented the basic
requirements for the development of the portable
device WS, operating on a number of effective
VOCs in the breath. Within the SEMEOTICON
Project, by the WS we intend a hardware/software
tool for both the analysis of VOCs of breath and a
platform for data mining and data integration.
Similar to a fingerprint, every individual has a
“breathprint” that can provide useful information
about his or her state of health. Thousands of unique
substances in exhaled breath have been identified.
These substances include elemental gases, such as
hydrogen, nitric oxide (NO), and carbon monoxide,
and a vast array of VOCs. The WS design has been
conceived to identify target VOCs with selective
sensors such as: nitric oxide, alcohols, carbon
monoxide and, possibly, other VOC substances
based on general requirement by physicians. In turn,
the breath gas sensors will be included in a box and
interacting with a main processing unit. In its
ultimate shape and functionality the WS will be a
compact and feasible device to be used in many
open environments and the most disparate situations
without losing efficiency.
ACKNOWLEDGEMENTS
Sara Colantonio, Paolo, Marraccini, Giuseppe
Coppini and Serena Detti are warmly acknowledged
for useful suggestions.
REFERENCES
World Health Organization. The Global Burden of
Disease: 2004 and update 2008.
Lison M. W.; Carl H.; Rafael P.; Dan L.; David N.; Mant
D., Glasziou P., 2010. What are the basic self-
monitoring components for cardiovascular risk
management?, MC Medical Research Methodology,
10:105.
Miekisch W.; Schubert J.; Nöeldge-Schomburg, G., 2004.
Breath analysis in critically ill patients: potential and
WizeSniffer-ANewPortableDeviceDesignedforSelectiveOlfaction
581

limitations, Expert Review Molecular Diagnosys , 4,
619-629.
Di Francesco F.; Fuoco R.; Trivella M.; Ceccarini A.
2005. Breath Analysis: trends in techniques and
clinical applications, Micromechanical Journal, 79,
405-410.
D’Amico, A.; Di Natale C.; Paolesse R.; Macagnano A.;
Martinelli E.; Pennazza G.; Santonico M.; Bernabei
M.; Roscioni C.; Galluccio G. et al., 2007. Olfactory
systems for medical applications, Sensors &
Actuators: B-Chemical, 130, 458-465.
Guo D.; Zhang D.; Li N.; Zhang L.; Yang J., 2007. A
Novel Breath Analysis System Based on Electronic
Olfaction, IEEE Transaction on Biomedical
Engineering.
Ding B.; Wang M.; Yu J.; Sun G., 2009. Gas Sensors
based on Electrospun Nanofibers, Sensors, 9, 1609-
1624.
Chen D.; Lei S.; Chen Y., 2011. A single Polyaniline
Nanofiber Field Effect Transistor and Its Gas Sensing
Mechanisms, Sensors, 11, 6509-6516.
Slater, J.; Watt, E.; Freeman, N.; May, I.; Weir, D., 1992.
Gas and vapor detection with poly(pyrrole) gas
sensors. Analyst, 117, 1265-1270.
Slater, J.; Paynter, J.; Watt, E., 1993. Multilayer
conducting polymer gas sensor arrays for olfactory
sensing. Analyst, 118, 379-384.
Savage, N.; Chwierogh, B.; Ginwalla, A.; Patton, B.;
Akbar, S.; Dutta, P., 2001. Composite n-p
semiconducting titanium oxides as gas sensors. Sens.
Actuat. B-Chem, 79, 17-27.
HEALTHINF2014-InternationalConferenceonHealthInformatics
582
