
An Improved Electrolytic Pump for Potential Drug Delivery
Applications
Ying Yi
1
, Ulrich Buttner
1
, Armando Arpys Arevalo Carreno
1
and Ian G. Foulds
2
1
Department of Electrical Engineering, King Abdullah University of Science and Technology (KAUST),
Thuwal, Saudi Arabia
2
Department of Electrical Engineering, University of British Columbia (UBC), Kelowna, Canada
Keywords: Electrolytic Pump, Platinum Mesh, Pumping Rate, Recombination Rate.
Abstract: This work presents a novel prototype of an electrolytic pump that uses a platinum (Pt) coated-nickel mesh in
the pumping chamber for increasing the recombination rate electrolysis products. The Pt coated-nickel mesh
that acts as a catalytic reforming element is able to significantly reduce the pulsed pumping period of the
pump, resulting in a more controllable system. Our presented electrolytic pump can provide the cyclical
actuation required for a solid drug in reservoir delivery system and shows potential application in the field
of drug delivery.
1 INTRODUCTION
The combination of microelectromechanical systems
(MEMS) with biochemical applications has attracted
growing interests, especially in the field of drug
delivery systems. MEMS fabrication technology
applied in drug delivery systems can provide a high
efficiency and accurately delivered dose and more
friendly operation (Nisar et al., 2008). MEMS based
drug delivery devices in general consisted of a
micro-pump, micro-fluid channels, micro-sensor and
another necessary units. As an essential component
of drug delivery devices, the micro-pump performs
the function of transferring the drug from the
reservoir to body tissue or blood vessel safely and
reliably. According to the different actuation
mechanism, micro-pumps can be divided into two
categories, namely mechanical micro-pumps and
non-mechanical micro-pumps.
In a mechanical micro-pump, actuators are
required to perform a pumping function. The
working mechanisms of mechanical micro-pumps
that show a potential application associated with
drug delivery systems are namely electrostatic
(Teymoori et al., 2005), piezoelectric (Junwu et al.,
2005), thermo-pneumatic (Hwang et al., 2005) and
shape memory alloy (SMA) (Guo et al., 2004).
Though mechanical micro-pumps provide a large
actuation, they require higher applied voltage and
consume more power causing large heat dissipation.
In contrast to mechanical pumps, non-mechanical
pumps do not need any reciprocating mechanical
component, the working principle of this kind
micro-pump is generally based on the conversion of
non-mechanical energy to kinetic momentum.
Moreover, non-mechanical micro-pumps usually
have neither moving parts nor complicated valves so
that the corresponding geometry design and
fabrication process are relatively simple. The
popular actuators that can be applied in non-
mechanical micro-pumps include osmotic-type (Su
et al., 2004) and electrochemical (Li et al., 2008 &
2010; Sheybani et al., 2012) categories. Osmotic-
type pump does not require any external power, but
its pumping force is extremely small and not
controllable. Therefore, osmotic pump is a suitable
option for those drug delivery applications of long-
term use with extremely low dosing requirements.
In contrast to those micro-pumps with the
traditional mechanisms, the electrolytic bubble
actuator has attracted a growing interest, particularly
in the field of drug delivery systems due to its easy
setup, low power consumption, miniaturized size,
simple operation, accurate delivery control and
adequate actuation force. In previously reported
electrolytic pumps for drug delivery (Li et al., 2008
& 2010), a liquid drug reservoir (LDR) approach has
been operated for high efficiencies, but requires
control of extremely small fluid volumes and
fabrication of complicated bellows. Recently, a solid
295
Yi Y., Buttner U., Arpys Carreno A. and G. Foulds I..
An Improved Electrolytic Pump for Potential Drug Delivery Applications.
DOI: 10.5220/0004943702950298
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2014), pages 295-298
ISBN: 978-989-758-013-0
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
drug in reservoir (SDR) approach was proposed
using magnetic actuation (Pirmoradi et al., 2011) to
pump fluid in and out of a reservoir that is filled
with a low solubility drug in solid form. This allows
for long term use because the reservoir can refill and
dissolve the next dose. This kind of micro-pump
shows a simplified structure but requires a strongly
aligned orientation and operates within short range.
Our proposed drug delivery system adopts the
simple structure of an SDR system and the
flexibility of an electrolytic pump system for
releasing the consistent drug. Moreover, improving
on our previous work (Yi et al., 2013), we further
introduce a platinum (Pt) coated nickel mesh into the
pumping chamber to achieve a higher recombination
rate, thereby reducing the pumping cycle time.
The rest of this paper is organized as follows;
Section 2 illustrates the prototype of the electrolytic
pump. Experimental setup and measurements are
evaluated in section 3. Conclusion is given in section
4.
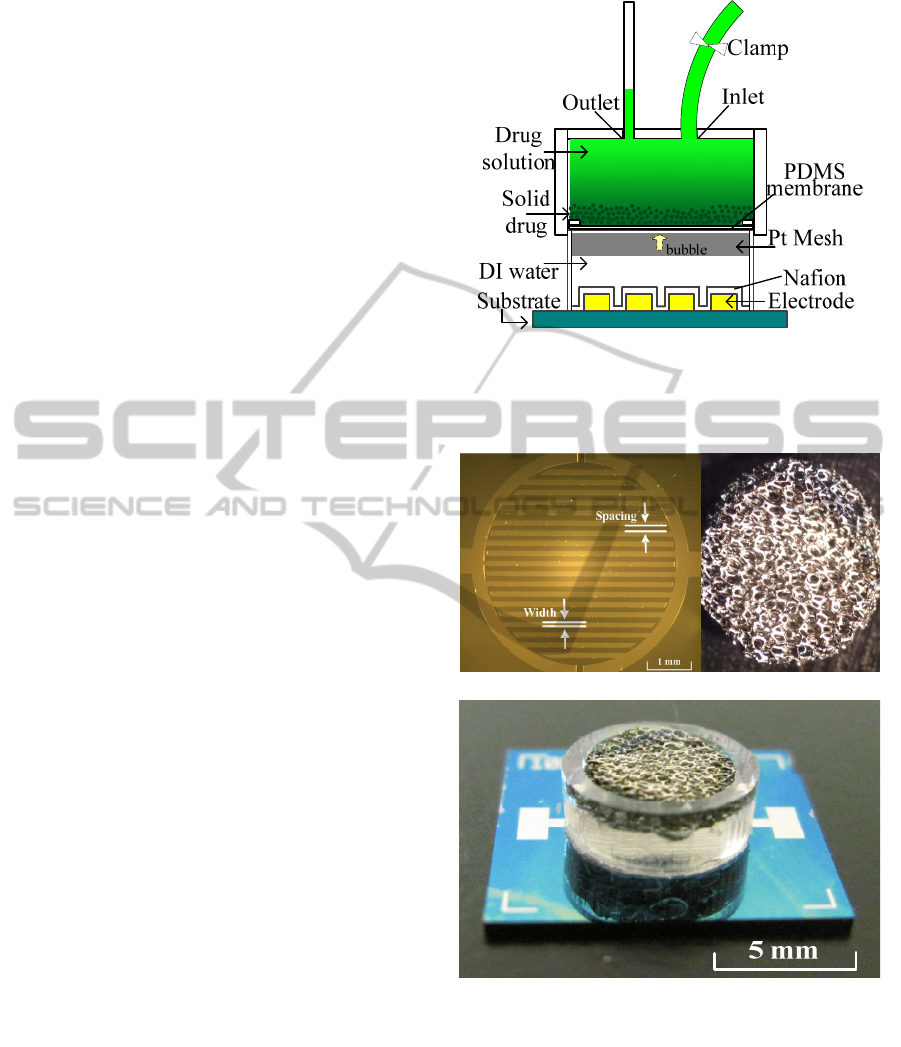
2 PUMP PROTOTYPE
This section illustrates a prototype of a drug delivery
system where the cyclical actuation is performed
using an electrolytic pump. Figure 1 illustrates a
cross sectional view of our initial prototype system.
For easy prototype testing, a cannula is replaced
by an inlet and outlet tube which can be
mechanically clamped. The major components of
our proposed electrolytic pump include: a flat PDMS
membrane, Pt electrode base and a Pt coated mesh.
The Pt electrode array was fabricated by a sputtering
technique and then patterned on a silicon wafer. The
corresponding design is shown in Figure 2 (a). The
dimensional parameters are 100 µm in width with
100µm spacing and a 400 nm height. Pt electrodes
are immersed in a deionized (DI) water filled
pumping chamber before assembling the other
components. Platinum was sputtered onto the
surface of a nickel mesh, forming a platinum coated
nickel mesh. Because the direction of sputtering is
vertical, the horizontal aspect of the nickel mesh was
not covered by platinum. When the Pt mesh was
immersed into the DI water, both platinum and
nickel were exposed to the electrolyte. The
corresponding micrograph of Pt mesh was shown in
Figure 2 (b). Figure 2 (c) illustrates the prototype of
pumping chamber. Nafion was coated onto the
platinum electrode to get a faster electrolysis-based
bubble generation rate (Sheybani et al., 2012). The
pumping chamber is separated from the drug
Figure 1: A cross-section view of the electrolytic pump
with major system components.
(a) (b)
(c)
(a) (b)
(c)
Figure 2: a) Micrograph of 400nm thick Pt electrode
layout showing element width and spacing of both 100μm.
b) Micro-scope of Pt-coated nickel mesh. c) Photograph of
pumping chamber with Pt mesh and Nafion coated
electrode.
reservoir by a PDMS membrane to avoid
electrochemical interaction with drug fluids. When
voltage is applied to the electrodes, electrolysis
reactions occur resulting in hydrogen (H
2
) and
oxygen (O
2
) bubble generation, the gas expansion
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
296
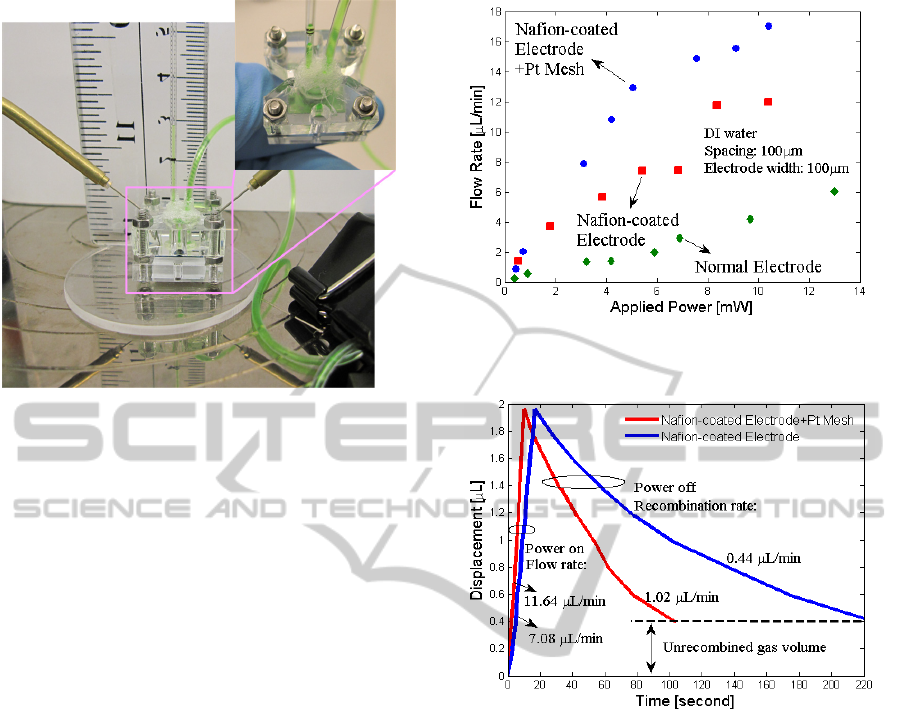
Figure 3: Photograph of the experimental setup and figure
of electrolytic pump.
pushes the membrane upwards to in turn push
dissolved drug solution out the cannula. Power is
applied until the desired volume of fluid is delivered,
after which the power is turned off and the pressure
in the electrolytic reservoir begins decreasing due to
the recombination of H
2
and O
2
. They rate of the
recombination is improved via the catalytic
properties of Pt, so that the membrane moves
downward drawing fresh fluids into the drug
reservoir to dissolve more of the remaining solid
drug. Power is turned on and off to the pump
periodically, so that the dissolved drug can be
delivered using this cyclical actuation.
3 EXPERIMENTAL RESULTS
Our proposed electrolytic pump was assembled and
measured using the test fixture as shown in Figure 3.
For easy prototype testing inlet tube is mechanically
clamped in order to accurately calculate the bubble
generation rate and recombination rate. The size of
the holder is 2 cm : 2cm : 2 cm (length : width :
height). In prospective drug delivery applications,
the pump could be permanently bonded allowing the
size of the structure to be significantly reduced. Two
probes are used to apply DC voltage to the
electrodes. A digital camera is placed in front of the
setup to record the displacement rate of the pump. In
the experiment, Nafion was uniformly spin-coated
onto electrodes because it is capable of preventing
bubble occlusion on the surface of the Pt electrode
and improving diffusion of gases away from the
catalyst surface (Maruyama et al., 1998).
Figure 4: Electrolytic pump flow rate vs. applied power
for with Pt-coated mesh and without Pt mesh.
Figure 5: Gas recombination rate comparisons for
electrolytic pumps with and without Pt mesh. Power of 4.6
mW was applied until the membrane achieved a maximum
displacement and then turned off.
Optimum electrode dimensions (Li et al., 2010) and
Nafion coating (Sheybani et al., 2012) had been
previously analyzed for achieving a higher pumping
efficiency, as shown in Figure 4. Based on the same
experimental conditions and electrodes, we added a
Pt mesh as shown in Figure 2 (b), and obtained a
higher flow rate than previous works (see Figure 4)
as well as a faster recombination rate (see Figure 5),
because Pt mesh increases the contact area between
the catalyst and electrolyte, improving catalytic
reactions.
Because the concentration of drug dose can be
kept stable during each delivery (Yi et al., 2013), a
faster bubble generation rate and recombination rate
that reduce each period of pumping allow delivering
a consistent and high drug volume within a short
pumping duration. Most importantly the Pt mesh
used in the electrolytic pump requires less power
AnImprovedElectrolyticPumpforPotentialDrugDeliveryApplications
297

(several mW) to achieve the same flow rate level
compared to the original electrolytic pump, which
makes the integration of wireless power transfer
techniques (Yi et al., 2013) and drug delivery
systems feasible.
4 CONCLUSIONS
This work presents a prototype electrolytic pump
that uses a Pt coated-nickel mesh in the pumping
chamber to improve the cycling time of an
electrolytic pump intended for a drug delivery
system. Using our catalytic reforming element, the
cyclical actuation of the drug delivery system
improved both in terms of the time of the
pump/recombination cycle, as well as the applied
power requirement, resulting in a faster and more
efficient drug delivery system.
REFERENCES
Nisar, A., Afzulpurkar, N., Mahaisavariya, B., &
Tuantranont, A. (2008). MEMS-based micropumps in
drug delivery and biomedical applications. Sensors
and Actuators B: Chemical, 130(2), 917-942.
Teymoori, M. M., & Abbaspour-Sani, E. (2005). Design
and simulation of a novel electrostatic peristaltic
micromachined pump for drug delivery applications.
Sensors and Actuators A: Physical, 117(2), 222-229.
Junwu, K., Zhigang, Y., Taijiang, P., Guangming, C., &
Boda, W. (2005). Design and test of a high-
performance piezoelectric micropump for drug
delivery. Sensors and Actuators A: Physical, 121(1),
156-161.
Hwang, S. R., Sim, W. Y., Kim, G. Y., Yang, S. S., &
Pak, J. J. (2005, May). Fabrication and test of a
submicroliter-level thermopneumatic micropump for
transdermal drug delivery. In Microtechnology in
Medicine and Biology, 2005. 3rd IEEE/EMBS Special
Topic Conference on (pp. 143-145). IEEE.
Guo, S., & Fukuda, T. (2004, April). SMA actuator-based
novel type of micropump for biomedical application.
In Robotics and Automation, 2004. Proceedings.
ICRA'04. 2004 IEEE International Conference on
(Vol. 2, pp. 1616-1621). IEEE.
Su, Y. C., & Lin, L. (2004). A water-powered micro drug
delivery system. Microelectromechanical Systems,
Journal of, 13(1), 75-82.
Li, P. Y., Shih, J., Lo, R., Saati, S., Agrawal, R.,
Humayun, M. S., ... & Meng, E. (2008). An
electrochemical intraocular drug delivery device.
Sensors and Actuators A: Physical, 143(1), 41-48.
Li, P. Y., Sheybani, R., Gutierrez, C. A., Kuo, J. T., &
Meng, E. (2010). A Parylene bellows electrochemical
actuator. Microelectromechanical Systems, Journal of,
19(1), 215-228.
Sheybani, R., & Meng, E. (2012). High-Efficiency MEMS
Electrochemical Actuators and Electrochemical
Impedance Spectroscopy Characterization.
Pirmoradi, F. N., Jackson, J. K., Burt, H. M., & Chiao, M.
(2011). A magnetically controlled MEMS device for
drug delivery: design, fabrication, and testing. Lab on
a Chip, 11(18), 3072-3080.
Yi, Y., Buttner, U., & Foulds, I. G. (2013, October).
TOWARDS AN IMPLANTABLE PULSED MODE
ELECTROLYTIC DRUG DELIVERY SYSTEM. In
Proc. Micro Total Analysis Systems.
Maruyama, J., Inaba, M., Katakura, K., Ogumi, Z., &
Takehara, Z. I. (1998). Influence of Nafion® film on
the kinetics of anodic hydrogen oxidation. Journal of
Electroanalytical Chemistry, 447(1), 201-209.
Yi, Y., Buttner, U., Fan, Y., & Foulds, I. G. (2013, May).
3-Coil resonance-based wireless power transfer system
for implantable electronic. In Wireless Power Transfer
(WPT), 2013 IEEE (pp. 230-233). IEEE.
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
298
