
Growth Mechanism of Rat Dorsal Root Ganglion Neurons
on Slope Substrate
Xiao Li, Yuanyuan Wang, Qi Xu, Fang Chen and Jiping He
Neural Interface and Rehabilitation Technology Research Center,
Huazhong University of Science and Technology, Wuhan, 430074, China
Keywords: Dorsal Root Ganglia (DRG), Axon, Slope Substrate.
Abstract: Neural response to topography depends on the dimensions and shapes of physical features. Most researchers
focused on fabricating different grooves and ridges to study cell adhesion, spreading, alignment, and
morphological changes. Very few papers report about how sloped substrate influences the behavior of
neural cells. In this paper, we made a preliminary experiment to test the reaction of neuronal growth
processes to different slopes. We found that all DRG cells’ axons couldn’t grow across 90 degree slope with
198 μm height. A few axons grew across 90 degree slope with 50 μm height. In addition, we also found that
DRG cells showed preference to grow uphill rather than downhill. In future, we will make more detailed
experiments to study the mechanism of slope modulation. This study will be helpful for the construction of
nerve regenerating scaffolds and neural interface.
1 INTRODUCTION
Every year, thousands of people are disabled by
neurological disease and injury. Successful control
of the cell behavior will open the ways for neural
regeneration and functional rehabilitation. Several
therapies offer significant promise for the restoration
of neuronal function, including the use of growth
factors to prevent cell death following injury
(Vincent and Feldman, 2002), stem cells to rebuild
parts of the nervous system (Horner and Gage,
2000), and the use of functional electrical
stimulation to promote axon regeneration (Al-
Majed, Neumann et al., 2000). During these
treatments, researchers have found that the
interaction of cells with substrate plays a key role in
the cell behavior, such as cell adhesion, spreading,
morphology, proliferation, and even differentiation.
Therefore, the research about how patterned
substrates influence the behavior of neurons appears
especially important right away.
The first experiment which mentioned the
relationship of cells and topography was
accomplished in 1911. Harrison (Harrison, 1911)
grew cells on a spider web and found the cells
followed the fibers of the web. Since then, with the
development of micro- and nano-fabrication
techniques, a large number of studies have shown
that many cell types react strongly to topography. In
general, micropattern substrates were fabricated to
contain repeating rectangular groove-plateau
patterns with varied groove width, varied plateau
width and varied groove depth. Neurons follow the
discontinuities of grooves
and ridges, and attain an
elongated shape due to surface-induced
rearrangements of the cytoskeleton (Curtis, 2004).
Actin and microtubules align along walls and edges,
the microtubules being the first element to be
aligned, followed by actin (Oakley and Brunette,
1993). Grooved surfaces also induce changes in
transcription and the up and down regulation of
several genes, but the explicit mechanism for cell
guidance has yet to be clarified (Dalby, Riehle et al.,
2003).
Most studies focused on the effects of
topography size on neurons. Rajnicek et al
(Rajnicek, Britland et al., 1997) reported that central
nervous system neurites could be guided by shallow
grooves with 14 nm deep and 1 mm wide. Stepien
and coworkers (Stepien, Stanisz et al., 1999)
reported contact guidance for chicken dorsal root
ganglion (DRG) neurons on single scratches with
0.1-0.2 mm wide. If the grooves are greater than 20
mm, no cell type (except red blood cells) has been
found to respond to the guide (Wilkinson, Riehle et
15
Li X., Wang Y., Xu Q., Chen F. and He J..
Growth Mechanism of Rat Dorsal Root Ganglion Neurons on Slope Substrate.
DOI: 10.5220/0005132600150020
In Proceedings of the 2nd International Congress on Neurotechnology, Electronics and Informatics (NEUROTECHNIX-2014), pages 15-20
ISBN: 978-989-758-056-7
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
al., 2002). Orientation often increases with
increasing depth, but decreases with increasing
groove width (Clark, Connolly et al., 1990). Goldner
et al (Goldner, Bruder et al., 2006) describe an
unusual capability of a subpopulation of DRG
neurons to extend neurites that spanned across the
grooves, with no underlying solid support. The
highest numbers of bridges observed under the
groove width of 30 mm, even few neurites bridge
have been observed spanning a groove of 200 mm.
Although these researches are important for
constructing high-resolution neural circuit scaffolds
or neural interface, the response of neurons and their
axons to the sloped substrata has not been studied in
detail. Fricke et al (Fricke, Zentis et al., 2011)
constructed a variety of gradient patterns with slight
changes in slope to control neuronal cell position,
the path of neurite growth, and axon directionality.
They found that reduction in the slope of structure
from 0.04 (0.3 mm/7.5 mm) to 0.01 (0.1 mm/7.5
mm) strongly decreased the effects on neurite
growth. However, because the slope substrate
consists of the multiple discontinuous grooves, we
can’t make sure that slope plays a key role in the
difference.
In this paper, we performed a preliminary
experiment to test the reaction of neuronal growth
processes at different slopes. The purpose of the
study was to investigate the preference of axons in
growing uphill or downhill on different angles of the
slope. Present results of this study can be utilized for
nerve regenerating scaffolds or the construction of
neural interface.
2 METHODS
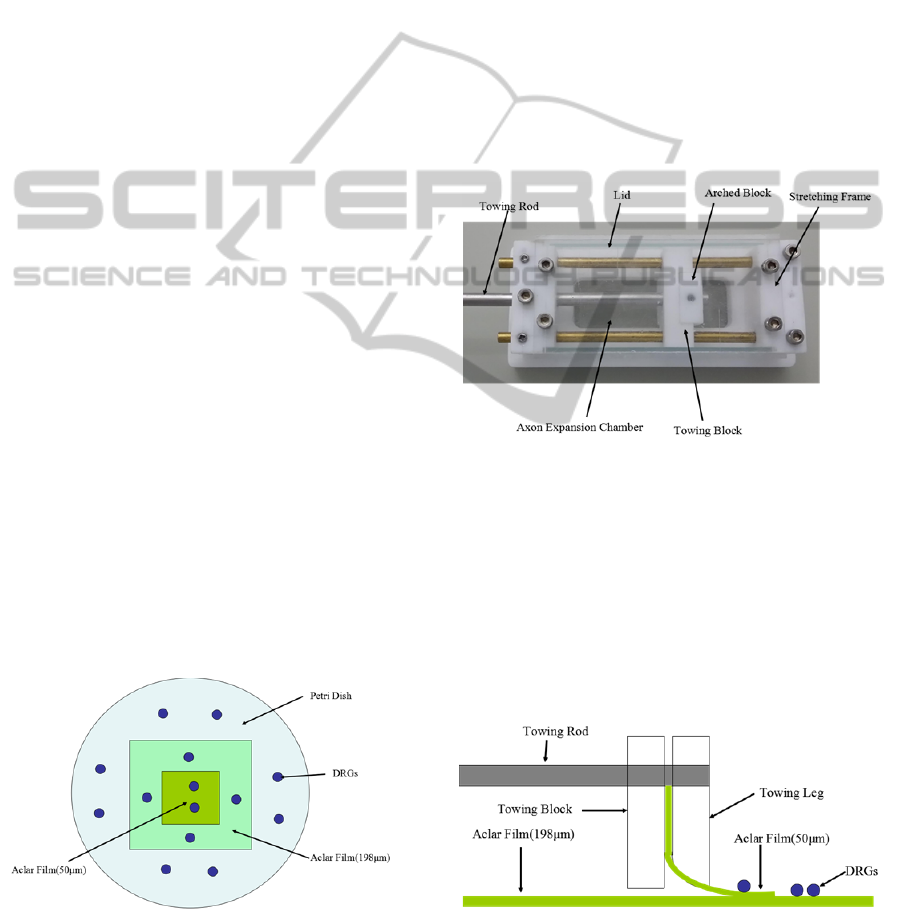
2.1 Construction of 90 Degree Slope
Figure 1: The schematic diagram of 90 degree slope
construction with different height. The powder blue disc is
Petri dish. The light cyan rectangular is 198 μm thick
Aclar film. The green rectangular is 50 μm thick Aclar
film. The blue dot is Dorsal root ganglia explants (DRGs).
The scheme of our experiment is illustrated in
Figure 1. Two Aclar (Aclar® 33C, Electron
Microscopy Sciences Inc., Hatfield, PA) films of
different thickness were plated in the Petri dish. The
dorsal root ganglia explants (DRGs) were plated on
the films or the bottom of Petri dish with different
locations. The 90 degree slopes with 50 or 198 μm
height were produced in this way.
2.2 Construction of Slight Degree Slope
We constructed an axon stretch-growth bioreactor
which contained two independent axon expansion
chambers. The axon expansion chamber consisted of
a stretching frame that formed a lane, an adjustable
towing block that could manipulate cells across the
lane, and a projected towing rod for external
manipulation (Figure 2).
Figure 2: Prototype of the axon expansion chamber.
Dorsal root ganglia (DRG) explants can be cultured in the
chamber.
The 198 μm thick Aclar film was affixed to the
bottom of the stretching frame and spanned the lane.
The 50 μm thick Aclar film was held rigidly by the
towing block. The 50 μm thick Aclar film was
lightly sanded on either side using 1200-grit
waterproof sandpaper (MATADOR, Germany) to
create gradual slope to the border of the exposed
underlying film (Figure 3).
Figure 3: The schematic diagram of slight degree slope
construction by axon stretch-growth system. Two different
Aclar films (green color) were used: one in the horizontal
plane and the other with a slight degree of slope. The blue
dot signifies Dorsal root ganglia explants (DRGs).
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
16
In order to get more convincing results, we could
move the towing rod (Figure 2 and 3) to divide the
culture by a computer-controlled micro-stepper
motor as described by Smith (Smith, Wolf et al.,
2001). This technique results in the stretch-induced
growth of fascicular tracts of axons spanning the two
membranes. Then we could measure the length and
diameter of the regular axons, which provided a
measure of the relevant ability of axons to climb the
slope substrate.
2.3 Cell Culture
Dorsal root ganglia (DRG) explants were isolated
from 1 day infant Sprague-Dawley rats (purchased
from the Wuhan University Center for Animal
Experiments) as described by Micevych et al.
(Chaban, Mayer et al., 2003). The experimental
protocol was approved by the Ethics Committee for
Animal Research, Huazhong University of Science
and Technology, China. Then dorsal root ganglia
(DRG) explants were plated in the containers as
shown in the Figure 1 and 3. The Aclar films were
washed with laboratory detergent, rinsed with
deionized water, sterilized in 70% ethanol, and
coated with 10 ng/mL high-molecular-weight poly-
D-lysine (Sigma, St.Louis, Mo) before the culture.
The culture medium was Dulbecco’s modified
Eagle’s medium (DMEM, HyClone, Logan, UT)
supplemented with 10% FBS (HyClone, Logan,
UT), 50 ng/ml nerve growth factor (rat β-NGF,
R&D, USA), and 1% penicillin/streptomycin.
Cultures were treated with the mitotic inhibitors
formulated with 10 mM 5-fluoro-2`-deoxyuridine
(FdU) (Sigma), and 10 mM uridine (Sigma) to
encourage non-neuronal cell elimination. The
incubation was conducted at 37 ℃ in a humid
atmosphere containing 5% CO
2
. The growth location
of DRG explants were observed on the Olympus
CKX41 inverted microscope (Olympus Inc. , Tokyo,
Japan), and recorded with a Canon 600D SLR
(Canon Inc., Tokyo, Japan).
3 RESULTS
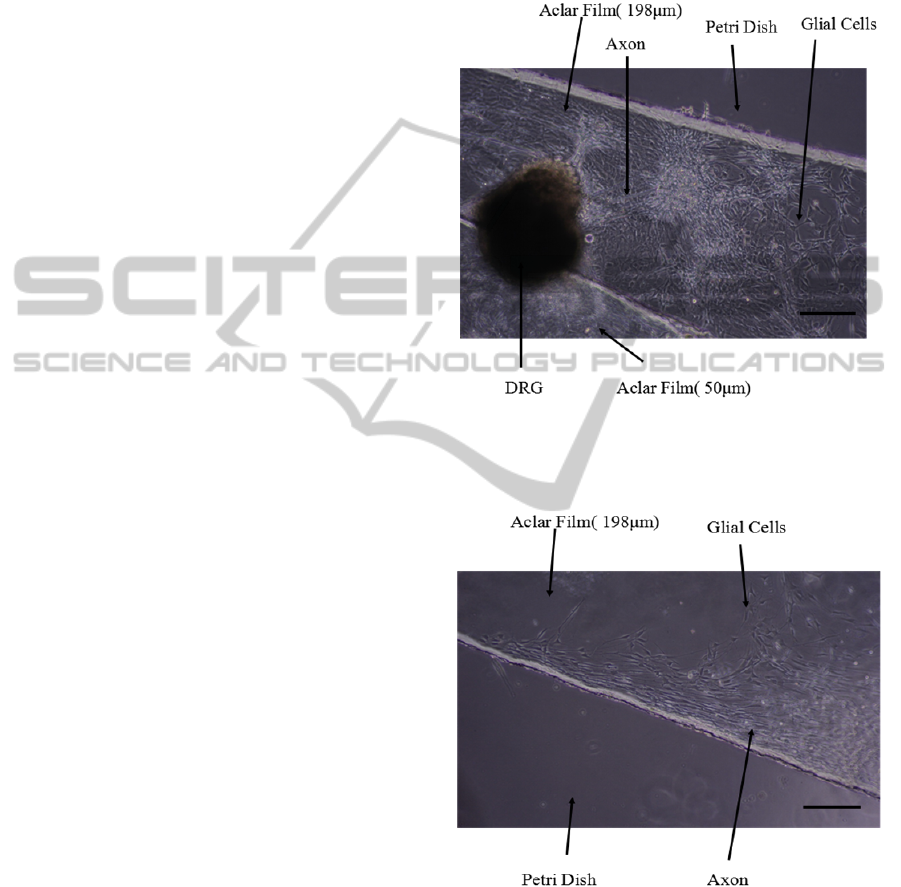
3.1 Cell Culture on 90 Degree Slope
After one day of incubation, a few glia cells could be
observed by microscope. After three days’
incubation, a large number of axons grew out from
the DRG explants following the path of glial cells
growth (Figure 5-8). Most of the DRG explants were
not in the defined position because the Aclar films
were shook when we changed the medium. Some
growth cones from the DRG explants advanced
more than one millimeter after 6 days (Figure 4).
However, they couldn’t grow down from the Aclar
film. Almost all axons and glial cells grew along the
edge of 198 μm thick Aclar film (Figure 5).
Figure 4: DRG explants cultured in the Petri dish with 90
degree slope. DRG neurite images were taken by a
10×objective, 6 days after the DRG explants were planted
on the Aclar strips. (Scale bar = 200 μm).
Figure 5: DRG explants cultured on 198 μm thick Aclar
film coated with PDL. DRG neurite images were taken by
a 10×objective, 3 days after the DRG explants were
planted on the Aclar strips. (Scale bar = 200 μm).
The morphology of axons grown on the 198 μm
thick Aclar film was obviously different from 50 μm
thick Aclar film (Figure 4 and 6). Therefore, most
axons didn’t grow down from 50 μm thick Aclar
GrowthMechanismofRatDorsalRootGanglionNeuronsonSlopeSubstrate
17
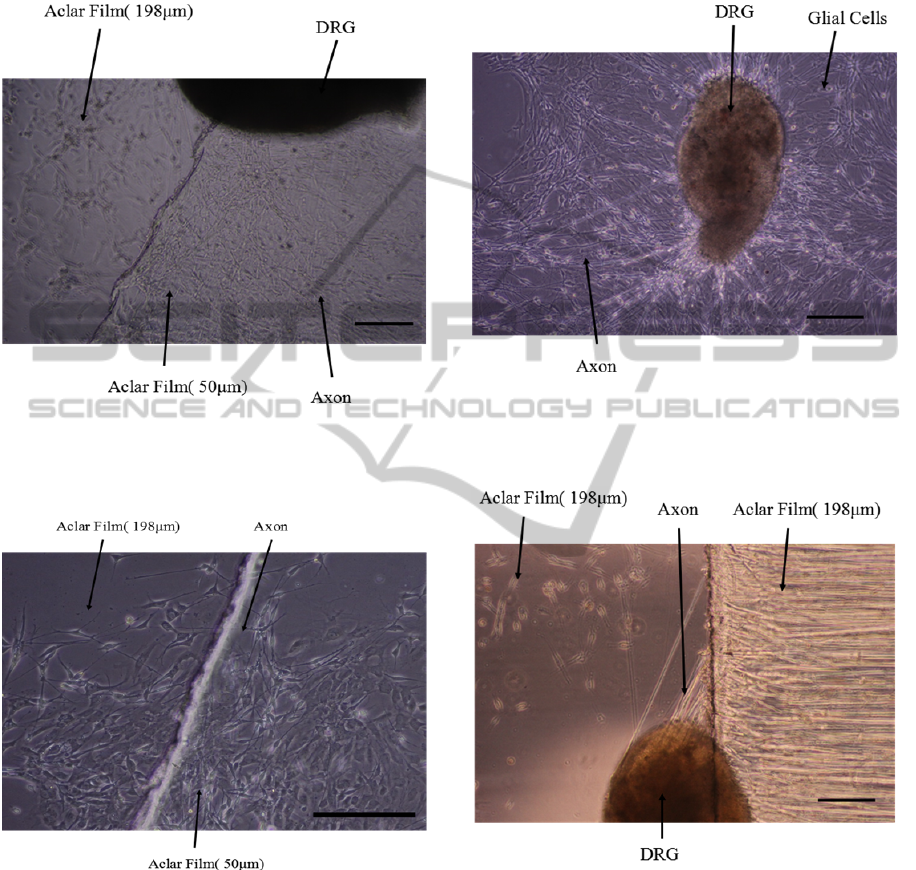
film to 198 μm thick Aclar film. A few axons grew
across the junction (Figure 7), but we could not
make sure that if these axons grew up from the 198
or 50 μm thick Aclar films.
Figure 6: DRG explants cultured on 50 or 198 μm thick
Aclar film coated with PDL. DRG neurite images were
taken by a 10×objective, 3 days after the DRG explants
were planted on the Aclar strips. (Scale bar = 200 μm).
Figure 7: DRG explants cultured on 50 or 198 μm thick
Aclar film coated with PDL. DRG neurite images were
taken by a 10×objective, 3 days after the DRG explants
were planted on the Aclar strips. (Scale bar = 200 μm).
On the bottom of Petri dish, a lot of axons and
glial cells migrated from the DRG explant (Figure 8).
The paths of axons growth were very clear with few
glial cells around the DRG explant. However, all
DRG explants were too far from the junction with
the 198 μm thick Aclar film to fail to arrive at its
edge. In order to make sure whether axons can grow
up 198 μm from 90 degree slope, we designed
another experiment by two pieces of 198 μm thick
Aclar film. Finally, we found that it is difficult for
axons to grow up on a 90 degree slope (Figure 9).
Figure 8: DRG explants cultured on the smooth bottom of
the Petri dish. DRG neurite images were taken by a
10×objective, 3 days after the DRG explants were planted
in the Petri dish. (Scale bar = 200 μm).
Figure 9: DRG explants cultured on 198μm thick Aclar
film coated with PDL. DRG neurite images were taken by
a 10×objective, 3 days after the DRG explants were
planted on the Aclar strips. (Scale bar = 200μm).
3.2 Cell Culture on Slight Degree Slope
In the axon stretch-growth bioreactor, a lot of axons
grew out from the DRG explants on the 50 μm thick
Aclar film (Figure 10). There were more axons on
the upper side than lower side. Previous studies have
shown that fibroblast cells prefer to grow uphill
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
18
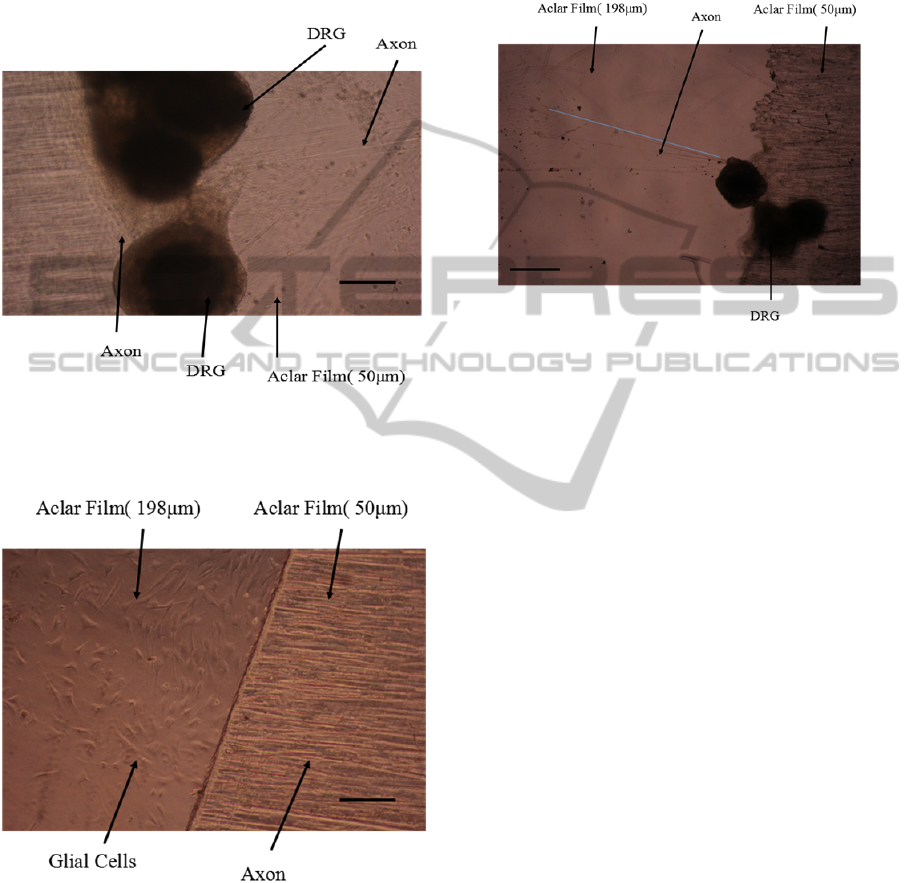
rather than downhill (Alaerts, De Cupere et al.
2001). In addition, Johansson et al found the axons
of the DRG cells on the original negative (grooved)
pattern were always found on the ridge edges, but
not in the grooves (Johansson, Carlberg et al. 2006).
This may explain why few axons grew down from
the 50 μm thick Aclar film (Figure 11).
Figure 10: DRG explants cultured on 50 μm thick Aclar
film coated with PDL. DRG neurite images were taken by
a 10×objective, 13 days after the DRG explants were
planted on the Aclar strips. (Scale bar = 200 μm).
Figure 11: DRG explants cultured on 50 or 198 μm thick
Aclar film coated with PDL. DRG neurite images were
taken by a 10×objective, 6 days after the DRG explants
were planted on the Aclar strips. (Scale bar = 200 μm).
After seven days’ culture in the axon stretch-
growth bioreactor, only a few of axons extended on
the bottom substrate. Stretch was applied by towing
the neuronal soma away from the growth cones by
taking a series of short 1
μm
steps. After two and a
half days’ stretch, the axons grew more than 1.75
mm and showed a regular alignment on the bottom
Aclar film (Figure 12). However, the axons didn’t
cross the 50 μm thick Aclar film, so we could not
assess the ability of stretched axons to climb on the
slope substrate.
Figure 12: DRG explants cultured on 50 or 198 μm thick
Aclar film coated with PDL. The blue line is more than
1.75 mm long. DRG neurite images were taken by a
4×objective, 10 days after the DRG explants were planted
on the Aclar strips. (Scale bar = 500 μm).
4 CONCLUSIONS
After nerve injury, neuronal connections are not
easily re-established. In the natural environment,
neurons are not growing on flat surface but in
complex three-dimensional microenvironment
formed by other cells or extra-cellular matrix. Along
with the chemical signals, neural behavior is also
determined by mechanical signals. As scarred tissue
is regenerates around the injury site, it is difficult for
the regenerating neurites to cross the injury gap. A
detailed analysis for the interaction of cells with
sloped substrate will not only support the
“regenerating axon” to cross the lesion in vivo but
also be helpful for the three-dimensional neural cell
cultures in vitro.
In this study, we performed a preliminary
experiment to test the reaction of neuronal growth
processes on the substrate slopes of different angles.
We found that all DRG cells’ axons couldn’t grow
across the 90 degree slope with 198 μm height. A
few axons grew across 90 degree slope with 50 μm
height. In addition, we also found that DRG cells
may also prefer to grow uphill rather than downhill.
This study didn’t provide the sufficient details
about the mechanisms which actually guide the
growth of neuronal cells on the different angles of
GrowthMechanismofRatDorsalRootGanglionNeuronsonSlopeSubstrate
19

substrate slopes, but it was reasonable to assume that
the guidance relied on extra-cellular cues, which
triggered some reorganization mechanisms in the
cytoskeleton. The actin cytoskeleton in cells
(fibroblasts, endothelia, and macrophages) reacting
to topography is organized in a way which we
believe to be appropriate for movement. Some
proteins, like semaphorines and ephrins, can inhibit
axons to grow the wrong way while other proteins
can attract axons to grow on the right way (Cook,
Tannahill et al. 1998). Compared with flat surface,
growth cones of the growing neurites on the slope
would get larger mechanical stress which can affect
the strength of the integrin–cytoskeleton links and
the integrin receptor distribution and conformation,
thus activating intracellular pathways active in cell
development and behaviour.
In future, we aim to make more experiments to
study the mechanism of slope modulation. For
example, we will put DRG explants on different
slope substrates to measure the growth rate of
neurites and observe the cytoskeleton morphology.
Even we will use quantitative real-time polymerase
chain reaction (qRT-PCR) to measure the magnitude
of changes in the expression of gene compliment
which regulates the neuron cell growth on different
topologies of substrates. Moreover, neurons are
usually not very likely to be the first cells to
encounter an implant as any topography may be
covered and obscured by glia cells (Franze 2013).
Therefore, we also need to study how slope affect
the glial cells.
ACKNOWLEDGEMENTS
This study was supported by the Natural Science
Foundation of China (grant number 61233015), the
National Basic Research Program of China (973
Program) (grant number SQ2012CB037202) and in
part by independent innovation fund of Huazhong
University of Science and Technology, Wuhan, P. R.
China (grant number 2013YGYL004). The authors
would like to thank Dr. Jun Ma and Dr. Jianfeng Xu
for their instruction in the experiments.
REFERENCES
Al-Majed, A. A., C. M. Neumann, et al. (2000). "Brief
electrical stimulation promotes the speed and accuracy
of motor axonal regeneration." The Journal of
neuroscience 20(7): 2602-2608.
Alaerts, J., V. De Cupere, et al. (2001). "Surface
characterization of poly (methyl methacrylate)
microgrooved for contact guidance of mammalian
cells." Biomaterials 22(12): 1635-1642.
Chaban, V., E. Mayer, et al. (2003). "Estradiol inhibits
ATP-induced intracellular calcium concentration
increase in dorsal root ganglia neurons." Neuroscience
118(4): 941-948.
Clark, P., P. Connolly, et al. (1990). "Topographical
control of cell behaviour: II. Multiple grooved
substrata." Development 108(4): 635-644.
Cook, G., D. Tannahill, et al. (1998). "Axon guidance to
and from choice points." Current opinion in
neurobiology 8(1): 64-72.
Curtis, A. (2004). "Small is beautiful but smaller is the
aim: review of a life of research." Eur Cell Mater 8:
27-36.
Dalby, M. J., M. O. Riehle, et al. (2003). "Nucleus
alignment and cell signaling in fibroblasts: response to
a micro-grooved topography." Experimental cell
research 284(2): 272-280.
Franze, K. (2013). "The mechanical control of nervous
system development." Development 140(15): 3069-
3077.
Fricke, R., P. D. Zentis, et al. (2011). "Axon guidance of
rat cortical neurons by microcontact printed
gradients." Biomaterials 32(8): 2070-2076.
Goldner, J. S., J. M. Bruder, et al. (2006). "Neurite
bridging across micropatterned grooves." Biomaterials
27(3): 460-472.
Harrison, R. G. (1911). "On the stereotropism of
embryonic cells." Science 34: 279-281.
Horner, P. J. and F. H. Gage (2000). "Regenerating the
damaged central nervous system." Nature 407(6807):
963-970.
Johansson, F., P. Carlberg, et al. (2006). "Axonal
outgrowth on nano-imprinted patterns." Biomaterials
27(8): 1251-1258.
Oakley, C. and D. Brunette (1993). "The sequence of
alignment of microtubules, focal contacts and actin
filaments in fibroblasts spreading on smooth and
grooved titanium substrata." Journal of Cell Science
106(1): 343-354.
Rajnicek, A., S. Britland, et al. (1997). "Contact guidance
of CNS neurites on grooved quartz: influence of
groove dimensions, neuronal age and cell type."
Journal of Cell Science 110(23): 2905-2913.
Smith, D. H., J. A. Wolf, et al. (2001). "A new strategy to
produce sustained growth of central nervous system
axons: continuous mechanical tension." Tissue
engineering 7(2): 131-139.
Stepien, E., J. Stanisz, et al. (1999). "Contact guidance of
chick embryo neurons on single scratches in glass and
on underlying aligned human skin fibroblasts." Cell
biology international 23: 105-116.
Vincent, A. M. and E. L. Feldman (2002). "Control of cell
survival by IGF signaling pathways." Growth hormone
& IGF research 12(4): 193-197.
Wilkinson, C., M. Riehle, et al. (2002). "The use of
materials patterned on a nano-and micro-metric scale
in cellular engineering." Materials Science and
Engineering: C 19(1): 263-269.
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
20
