
Pilot Testing of a Computer-Aided Prevention System (CAPSYS)
Study Protocol of a Randomized Controlled Trial
Lübomira Spassova
1
, Norbert Rösch
2
, Debora Vittore
3
and Dirk W. Droste
3
1
Public Research Centre Henri Tudor, SANTEC, 29, avenue John F. Kennedy, 1855, Luxembourg, Luxembourg
2
University of Applied Sciences Kaiserslautern, Amerikastr. 1, 66482, Zweibrücken, Germany
3
Centre Hospitalier de Luxembourg, Department of Neurology, 4, rue Ernest Barblé, 1210, Luxembourg, Luxembourg
Keywords: Lifestyle Coaching, CVD Risk Factors, CVD Prevention, Randomized Controlled Trial.
Abstract: The CAPSYS system enables automated lifestyle coaching for CVD patients through a phone-based
interface with the aim of establishing a healthy behavior by reducing specific risk factors. This paper
presents the procedure of a randomized controlled trial currently performed to evaluate the CAPSYS
prevention approach in terms of efficacy and usability.
1 BACKGROUND
Cerebro-cardiovascular diseases (CVDs) – especial-
ly heart disease and stroke – are the leading causes
of mortality worldwide, and it is estimated that the
annual mortality rate for CVDs will even increase in
the coming decades (WHO, 2013). According to the
WHO, unhealty diet, obesity, physical inactivity,
high blood pressure and tobacco use are among the
main risk factors for CVDs. Thus, to prevent heart
disease and stroke, affected persons are advised to
engage in regular physical activity, to avoid tobacco
smoke, to achieve and maintain a normal body
weight, and to stick to a healthy diet rich in fruit and
vegetables, avoiding too much fat and sugar. It could
be shown in several studies and epidemiologial
analyses that the risk of stroke or myocardial
infarction can be reduced through a healthy diet and
regular physical activity (Droste, 2013).
The Computer-aided Prevention System
(CAPSYS) has been developed by health scientists
and IT experts in collaboration with neurologists
with the aim to support patients in the primary and
secondary prevention of CVDs (Spassova, 2013).
This system allows patiens to provide information
on their individual CVD-related risk factors through
a phone-based interface. Based on this data, each
patient receives system-generated customized
feedback on his or her progress and advice on how
to proceed reducing the risk factors that are still in a
critical range. Based on recommendations by the
Luxembourg Medical Scociety, this computerized
processing of patient data allows automated
personalised lifestyle coaching of patients in real
time. The attending physician can monitor the
progress of each patient through a secure web
interface offering a graphical representation of the
development of the corresponding risk factors (see
Figure 1).
The CAPSYS lifestyle coaching approach is
currently evaluated in a randomized controlled trial
(RCT) in terms of efficacy in reducing the
concerned risk factors as well as regarding its
usability and user acceptance.
2 RELATED WORK
In a parallel two-arm RCT with 171 participants
with ischemic heart disease, Maddison et al.
investigated the efficiency of a mobile phone and
internet-based cardiac rehabilitation program, which
was delivered for six months and consisted of
physical exercise prescription and behavioral change
support in form of text messages sent to the
participants of the intervention group (Maddison,
2011). Although no statistical difference could be
observed for the primary outcome, which was peak
oxygen uptake (PVO
2
), significant differences were
found concerning physical activity, task efficacy and
motivation as well as for the general health domain
of the SF-36 health-related quality of life measure in
favor of the intervention group (Carter, 2013).
50
Spassova L., Rösch N., Vittore D. and Droste D..
Pilot Testing of a Computer-Aided Prevention System (CAPSYS) - Study Protocol of a Randomized Controlled Trial.
DOI: 10.5220/0005142800500054
In Proceedings of the 2nd International Congress on Cardiovascular Technologies (CARDIOTECHNIX-2014), pages 50-54
ISBN: 978-989-758-055-0
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
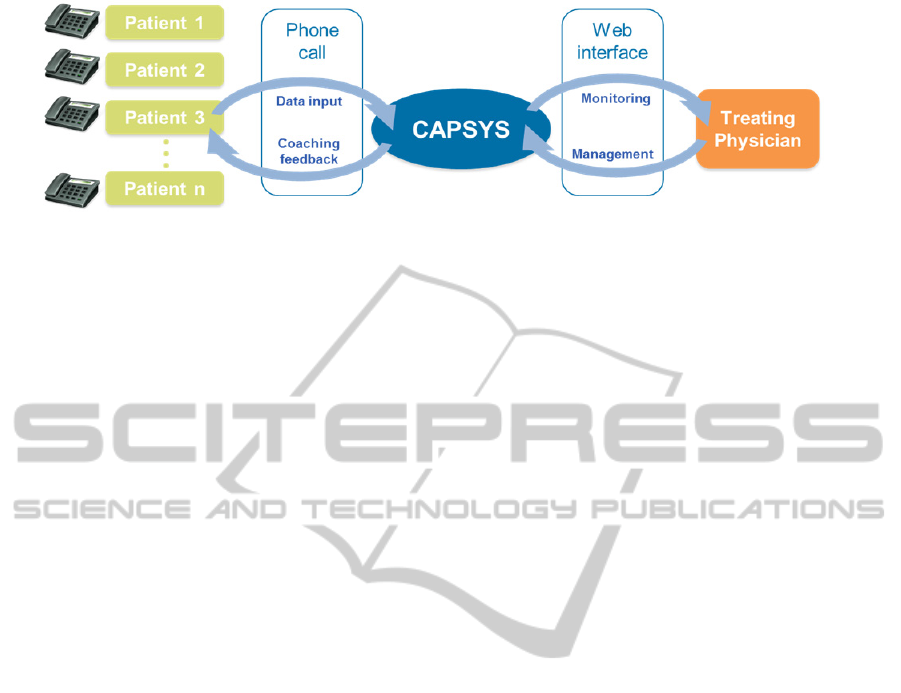
Figure 1: Architecture of the CAPSYS system.
In another study, Pfaeffli Dale et al. evaluated the
efficacy of a 4-week healthy eating program in
achieving behavior change among 20 CVD patients
with high blood cholesterol (Pfaeffli Dale, 2014).
The modality of the intervention was very
similar to the one applied in the Maddison study, i.e.
text messages providing support for behavioral
change received through mobile phones or through a
website. However, in this case the aimed
behavioural change consisted in establishing a
healthy diet. The results revealed some post-
intervention increase in perceived self-efficacy
concerning heart-healthy eating.
Walters et al. have developed a mobile phone
and web-based care model for outpatient cardiac
rehabilitation, and they have evaluated this approach
in a 6 week program (Walters, 2010). The system
provides daily motivational messages sent through
SMS and weekly mentoring by human assistants
through video conferencing. These mentoring
sessions involve the setting and assessment of
specific goals concerning exercise and behavior
modification. Some factors, such as movement
activity, are automatically measured through
sensors. Results show significantly higher adherence
rates, larger walking distances, weight reductions
and improved emotional states in the intervention
group as compared to the control group (Walters,
2012).
These empirical results support the assumption
that telephone-based lifestyle coaching can lead to
an improvement in health behavior in the CVD
context. According to the Fogg Behavior Model,
human behavior is based on the presence of three
fundamental factors: motivation, ability and triggers
(Fogg, 2009). In the proposed CAPSYS setting, the
phone-based interventions provide motivational
feedback and at the same time build the triggers for
positive behavior change. The ability of the users to
participate in the coaching intervention is supported
by the simplicity of the phone-based interface
(Rösch, 2013).
3 STUDY DESIGN
The CAPSYS study has been implemented as a
parallel two-arm RCT with the following criteria.
Inclusion criteria:
Age: from 20 with no upper limit;
Patients who already suffered a stroke or
Transient Ischemic Attack (TIA);
Patients with at least two increased risk factors
for stroke:
o High blood pressure
o Overweight
o Low physical activity
o Smoking
o Unhealthy diet
Exclusion criteria:
Inability to fill out or to understand the informed
consent;
No signed informed consent;
Dementia
Participants are contacted and screened for
eligibility by the attending neurologist during
medical consultations. After signing an informed
consent, patients are randomly assigned either to the
standard care group (SC) or to the interventional
care group (IC). A total of 94 patients have been
recruited (SC: 46, IC: 48). At the beginning and at
the end of the 6-month study period, the participants
from both goups are asked to complete a
questionnaire on demographic and medical data,
including:
Medical history (stroke, TIA, diabetes etc.)
Current medication
Risk factors:
o Smoking habits
o Body weight, height ( BMI)
o Blood pressure, heart rate
o Blood test results (HDL, LDL, HbA1c,
glycemia and triglyceride levels)
In addition, three further questionnaires are to be
completed also at the beginning and at the end of the
PilotTestingofaComputer-AidedPreventionSystem(CAPSYS)-StudyProtocolofaRandomizedControlledTrial
51

study period to collect data about the nutritional
habits, the physical activity and the quality of life of
the participants from both groups.
With the questionnaire on nutritional habits,
participants can self-assess their usual daily or
weekly food consumption at baseline and post-
intervention. The questionnaire encompasses a
number of food groups such as fish, meat, dairy,
fruits, vegetables, sweets, alcohol etc.
The questionnaire on physical activity asks for a
self-assessment of the following types of activity (in
hours and minutes) that the participants usually
perform per week:
Low intensity: normal breathing, no sweating
Moderate intensity: slightly accelerated
breathing, potentially some sweating
High intensity: rapid breathing and sweating
Finally, information about the participants’
quality of life is acquired by means of the
standardized EQ-5D-5L instrument provided by the
EuroQol Group (The EuroQol Group, 1990).
At the beginning of the study, the participants are
assisted by an interviewer (a study nurse, an
assistant doctor or the project staff) in filling in the
questionnaires. The blood pressure and the heart rate
of each participant are measured by the interviewer
at the beginning and at the end of the study period.
Data concerning the participants’ medical history,
current medication and blood test results are taken
from the corresponding medical files. At the end of
the recruitment meeting, patients of both groups are
handed out a leaflet with information on healthy
lifestyle, especially concerning nutrition and
physical activity as guidance for reducing their risk
factors.
Patients in the IC group are assigned a personal
account (patient number and PIN code) for accessing
the CAPSYS system. The interviewer demonstrates
how to use the CAPSYS system and provides a
paper-based user guide with detailed instructions on
the application. The IC participants are advised to
call the CAPSYS system twice a week (preferably
on Mondays and Thursdays).
During the study period, SC participants do not
undergo any special treatment, but they receive the
usual care by their treating physicians. In addition to
the usual medical care, IC participants are provided
the possibility to use the CAPSYS system in order to
provide information about their current lifestyle
concerning certain CVD risk factors and to receive
feedback on the provided values by the system (see
Figure 2). The participants can access the system by
calling a land line number with their stationary or
mobile phones. After they have chosen their
preferred language (French or German) and
authenticated themselves with their patient number
and PIN code, the participants are posed some
automatically generated questions concerning their
current blood pressure and body weight, and about
their physical activity and their consumption of
fruits, vegetables, whole-grain food and sweets on
the previous day. For smokers, cigarette
consumption is also considered.
During the recruitment meeting at the beginning
of the study, participants are instructed on how to
estimate portion sizes for the relevant food groups,
and they are advised to measure their current blood
pressure and body weight, and to write down all
necessary values prior to calling the CAPSYS
system. The system is implemented to run fully
automatically and can be accessed at any time of
day. The questions and feedback generated by
CAPSYS are issued to the users in spoken natural
language by means of text-to-speech software
(TTS). The participants can answer to the questions
by typing in corresponding numerical values using
the phone key pad (e.g. “3” for 3 portions of fruits
Figure 2: Outline of a CAPSYS phone call.
CARDIOTECHNIX2014-InternationalCongressonCardiovascularTechnologies
52

and vegetables, or “79” for a body weight of 79 kg).
All provided values are automatically saved in a
secure database for later evaluation.
At the end of their study participation (approx.
one month before the end), participants are
contacted by letter requesting them to fill in the final
questionnaires as far as possible (except for medical
data) and to bring them to their next appointment
with the attending neurologist. If participants need
support in completing the finalization
questionnaires, they are supported by an interviewer,
who also measures their blood pressure and heart
rate and fills in the required medical data. In
addition to the finalization questionnaires, which are
almost identical to the ones completed at the
beginning of the study (nutrition, physical activity
and quality of life), IC participants are asked to fill
in one additional form with questions concerning the
usability of the system and the user satisfaction. This
questionnaire encompasses the standardized System
Usability Scale (SUS) (Brooke, 1996) and a number
of system-specific questions, e.g. concerning the
system features (quality of the TTS voice, adequacy
of the automated feedback etc.), the preferred
frequency of use and the perceived benefits after 6
months of usage.
Taking into account the bilingual setting of the
study site, all questionnaires as well as the CAPSYS
system itself are available in French and German.
Both at the beginning and at the end of the study,
data are collected through paper questionnaires and
saved in electronic form in a dedicated secure
database.
The CAPSYS study has been approved by the
Luxembourg National Research Ethics Committee
(CNER) (N° 201205/08) and the National
Commission for Data Protection (CNPD)
(T007990).
4 EVALUATION APPROACH
The main objective of the CAPSYS study is the
evaluation of measurable clinical effects of
computer-supported lifestyle coaching in patients
with increased CVD risk factors. Consequently, the
primary endpoint of the study is the change of CVD
risk factors over time in both study arms.
Questionnaire data (q-data) and measurements
(m-data) of physiological parameters (blood
pressure, BMI, HDL, LDL, HbA1c
and glycemia)
are collected at baseline and post-intervention.
Individual risk profiles and patterns of patients will
be generated and analyzed based on official
recommendations by the EACPR (European
Association of Cardiovascular Prevention and
Rehabilitation) (Perk, 2012). Data will be analyzed
using the Student’s t-test approach (Hazewinkel,
2001). Paired t-tests will be applied to find
significant changes in the physiological parameters
between baseline and post-intervention. Unpaired
two-sample t-tests for each parameter can reveal
possible significant differences in increase or
decrease of risk factors between the two groups.
Statistical analyses will be performed using the R
environment, with a significance level of 0.05.
As secondary endpoint, health-related quality of
life (HR-QoL) will be analyzed. The quality of life
data collected through the standardized EQ-5D-5L
questionnaire (health profiles and values of the EQ
visual analogue scale (EQ VAS)) will be
summarized in appropriate charts according to the
EuroQol guidelines.
The usability of the CAPSYS system will be
evaluated based on the results of the SUS form
(standardized numeric evaluation schema) and the
answers to the additional system-specific questions
provided by the IC participants.
5 CONCLUSIONS
The study presented in this article aims at evaluating
the efficacy and usability of the CAPSYS approach
for phone-based lifestyle coaching of CVD patients
concerning risk factor reduction. The results of this
study will be presented in a separate publication
upon study finalization. In case the results provide
proof of clinical impact, the health economic aspects
of the proposed approach will need to be further
investigated. Potential implementation and
maintenance costs concerning the incorporation of
CAPSYS into CVD healthcare will be analyzed and
compared to those of traditional and alternative
CVD prevention approaches. Based on the usability
evaluation results, the CAPSYS system will be
further refined and adapted to the users’ needs.
REFERENCES
WHO, Cardiovascular diseases (CVDs), Fact sheet No.
317, World Health Organization, 2013
Droste, D.W., Keipes, M., 2013. (The reduction of stroke
risk, risk of myocardial infarction and death by healthy
diet and physical activity.) In Bulletin de la Societe des
Sciences Medicales du Grand-Duche de Luxembourg,
2013(2):51-62.
PilotTestingofaComputer-AidedPreventionSystem(CAPSYS)-StudyProtocolofaRandomizedControlledTrial
53

Spassova, L., Rösch, N., Vittore, D., Droste, D.W., 2013.
CAPSYS: Computer-aided Prevention through
Automated Lifestyle Coaching. In Proceedings of
IADIS International Conference on e-Health, pp. 169–
173.
Spassova, L., Vittore, D., Droste, D., Rösch, N., 2013.
Automated lifestyle coaching for cerebro-
cardiovascular disease prevention. In Studies in Health
Technology and Informatics, vol. 190, pp. 234–236.
Maddison, R., Whittaker, R., Stewart, R., Kerr, A., Jiang,
Y., Kira, G., Carter, K.H., Pfaeffli, L., 2011. HEART:
heart exercise and remote technologies: A randomized
controlled trial study protocol. In BMC
Cardiovascular Disorders 2011, 11:26
Carter, K., Maddison, R., Whittaker, R., Stewart, R., Kerr,
A., Jiang, Y., Pfaeffli, L., Rawstorn, J., 2013. Heart:
Efficacy of a mHealth Exercise-based Cardiac
Rehabilitation Program. In Heart, Lung and
Circulation, 07/2013, 22(7):553.
Pfaeffli Dale, L., Whittaker, R., Eyles, H., Ni Mhurchu,
C., Ball, K., Smith, N., Maddison, R., 2014.
Cardiovascular Disease Self-Management: Pilot
Testing of an mHealth Healthy Eating Program. In
Journal of Personalized Medicine, 19 March 2012,
4(1), 88-101.
Walters, D.L. et al., 2010. A mobile phone-based care
model for outpatient cardiac rehabilitation: the care
assessment platform (CAP). In BMC Cardiovascular
Disorders 2010, 10:5.
Walters, D.L. et al., 2012. Technology Based Home-Care
Model Improves Outcomes Of Uptake, Adherence
And Health In Cardiac Rehabilitation. In Heart, Lung
and Circulation 2012, 21:S143–S316.
Fogg, B.J., 2009. A Behavior Model for Persuasive
Design. In Proceedings of the 4th International
Conference on Persuasive Technology, 40:1-7.
Rösch, N., Spassova, L., Vittore, D., Droste, D.W. , 2013.
(Computer-aided Lifestyle Coaching complements
telemedical control of cerebro-cardiovascular risk
factors). In Nutzung, Nutzer, Nutzen von Telematik in
der Gesundheitsversorgung – eine Standort-
bestimmung, pp. 37–43, 2013
The EuroQol Group, 1990. EuroQol – a new facility for
the measurement of health-related quality of life.
Health Policy 16(3):199–208.
Brooke, J., 1996. SUS: a "quick and dirty" usability scale.
In P. W. Jordan, B. Thomas, B. A. Weerdmeester, and
A. L. McClelland. Usability Evaluation in Industry.
London: Taylor and Francis.
Perk, J. et al., 2012. European Guidelines on
cardiovascular disease prevention in clinical practice
(version 2012). In European Heart Journal (2012) 33,
1635–1701.
Hazewinkel, M., 2001. Student test, Encyclopedia of
Mathematics, Springer, ISBN 978-1-55608-010-4.
CARDIOTECHNIX2014-InternationalCongressonCardiovascularTechnologies
54
