
Multi-biosignals Analysis
The Effect of Peripheral Nerve Stimulation on Skin Conductance and Heart Rate
Variability
Tiago Araújo
1
, Pedro Dias
1
, Neuza Nunes
2
and Hugo Gamboa
1
1
Department of Physics, Faculty of Sciences and Technology, New University of Lisbon, Lisbon, Portugal
2
PLUX - Wireless Biosignals S.A., Lisbon, Portugal
Keywords: Skin Conductance, Blood Volume Pulse, Heart Rate Variability, Peripheral Nerve Stimulation.
Abstract. Objectives: This study aims to evaluate the influence of standard electrical stimulation on human
electrophysiology. Methods: A total of 10 healthy subjects were submitted to the same protocol. The
electrical stimuli were applied on the median nerve of the left wrist. Blood Volume Pulse (BVP) and
Electrodermal Activity (EDA) signals were acquired from the index finger through an oximeter and from
both the abductor pollicis muscle and the 3
rd
palmar interosseous muscle of the right hand, respectively.
Nerve stimulation was performed using increasing intensities current: range from 5 to 30 mA, with 1mA
step and applying 20 stimuli per step. Heart Rate (HR) and Heart Rate Variability (HRV) were computed,
from the analysis of the latency between BVP pulses, in basal state and during stimulation. EDA parameters
response latency, response rise time and readaptation slope were computed for each burst.
Discussion: Electrical stimulation reveals to influence several parameters of the Autonomic Nervous System
(ANS). It was easily detected an EDA rise response for each of the applied bursts and also an increase of the
HRV during stimulation.
1 INTRODUCTION
Recently, the importance of studying biosignals has
been increasing due to its role finding physical and
mental stress. It has already been determined that the
Autonomic Nervous System (ANS) exerts a constant
influence over heart rate (HR) and skin conductance.
Despite all the studies, there has always been a gap
in the understanding of the effects of electrical
stimulation in human physiology (Shetter, A., 1997;
Dimitrijevic, M., 2008).
Skin Conductance (Electrodermal Activity) has
been reported as a potential non-invasive marker for
sympathetic activity, and has been used recently in
psychophysiological research. The Electrodermal
activity (EDA) measurement is based on content of
water and electrolytes in individual parts of the
organism and the spreading of low electrical current
through two electrodes localized on skin surface.
The conductance depends on the amount of
sweat produced by eccrine glands, which are
regulated by the sympathetic nervous system
(Visnovcova, 2013). It’s the sweat level changes that
modify the resistance, and alterations in the EDA
signal are noticed.
The total EDA is composed of a baseline and a
phase level. The baseline is the EDA on a daily
basis. Providing the EDA is not a constant value it
was noted that its oscillations are very small over a
small time interval. Therefore we considered the
EDA baseline to be linear. The phase level is a
variation of a subject’s EDA due to external stimuli
applied on the wrist and reflects the arousal of the
sympathetic nervous system (Dominik, B., 2010).
The EDA parameters usually analysed are the
response latency, response rise time and readaptation
slope.
EDA latency is the time interval between the
application of the first stimulus and the detection of
a response from the subject’s EDA. EDA rise time is
the time taken by an EDA response to rise and peak.
Finally, EDA readaptation slope is defined as the
slope of the line obtain from a linear regression with
all data points past the last stimulus.
The heart rate is very sensitive and readapts
quickly to different stimuli. The sympathetic and
parasympathetic neurons, which are linked to the
sinoatrial node in the heart, have a major
contribution to changes in its beating rate (although
there are more contributions, like the physical and
235
Araújo T., Dias P., Nunes N. and Gamboa H..
Multi-biosignals Analysis - The Effect of Peripheral Nerve Stimulation on Skin Conductance and Heart Rate Variability.
DOI: 10.5220/0005217902350239
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2015), pages 235-239
ISBN: 978-989-758-069-7
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

mental state). Heart rate variability (HRV) is the
physiological phenomenon in which the heart rate
oscillates around its mean value due to external
influences from the autonomic nervous system
(ANS), or other mental and physical factors (Malik,
M., 1990; Malik, M., 2006). It’s a marker to study
the activity of the regulatory mechanisms and to
analyse the effects (excitatory and inhibitory) they
have upon the heart rate.
HRV can be analysed on time or frequency
domains. Through the analysis of the root mean
square of the
successive RR intervals we can obtain a
measure of the sympathetic and parasympathetic vagal
activity (Clifford, G., 2002). One way to measure the HR
is through the Blood Volume Pulse (BVP) sensor. With
the cardiac pulse, erythrocytes will change the spatial
alignment and this will affect the blood’s opacity. The
BVP sensor makes use of opacity variations from the tip
of the finger to gather the cardiac pulse on a certain
instant.This study
aims to analyse the effects of
electrical stimulation on human physiology, based
on skin’s galvanic response and on Blood Volume
Pulse (BVP) signals.
2 METHODS
2.1 Subjects and Room Conditions
In this study a total of 10 healthy subjects, composed
of 5 males and 5 females, with a mean age of 24.10
years (standard deviation of 2.38 years), were
submitted to a previously developed heart rate and
skin conductance acquisition protocol. All of the
subjects were healthy, with no physical or
neurophysiological disorders registered.
The subjects were seated on a comfortable chair
with both their arms relaxed. It was required of them
to feel comfortable for the acquisition to begin.
Earmuffs were placed on the subjects’ ears to
prevent any noise distraction. Moreover the room
was kept silent during the acquisition and the
subjects were asked to remain motionless and
relaxed.
The stimuli were applied through two disposable
electrodes on the median nerve of the left wrist and
two electrodermal activity acquisition electrodes
were placed on the abductor pollicis muscle and on
the 3
rd
palmar interosseous muscle of the right hand.
An oximeter was also placed on the index finger of
the right hand to measure the heart rate of the
subjects. The sensor makes use of colour variations
from the tip of the index finger (due to blood’s
opacity) to gather the blood volume on a certain
instance in time.
Although the majority of the subjects didn’t
know about the acquisition protocol, the acquisition
had to be repeated on two of the subjects (1 and 2)
due to low quality signal reading.
2.2 Acquisition Protocol
The first part of the acquisition took 4 minutes and
no stimuli were applied to the subjects. This segment
of the acquisition protocol had the purpose of
acquiring the basal BVP and EDA.
The second segment featured the application of
the electrical stimuli on the medial nerve of the left
wrist. Square current modulated waveform was
used. The electrical stimuli consisted of 6 bursts of
20 repetitive stimuli each and with increasing
intensities (ranging from 5 to 30 mA). In each burst
the interval between stimuli was 0.9 seconds and the
time interval between bursts was of 10 seconds.
The final segment of the acquisition was similar
to the first one, a 4-minute acquisition to record the
subjects’ recovery after the application of stimuli.
Through all of the phases, these segments were
recorded at a 2000Hz acquisition rate. A combined
wireless, miniaturized and synchronized unit was
specifically developed for multi-biosignal
acquisition (Plux, 2014) and nerve stimulation
(Araújo, T., 2012).
2.3 Processing
After the acquisition from all subjects the data was
processed using a script in Python. The data was
stored in a .txt file (for each subject) and organized
in columns, each column belonging to a certain
parameter and each line to a recorded frame.
The data recorded was processed according to
steps bellow:
1. Conversion of frames to seconds, since 1000
frames corresponded to a single second.
2. Search for all HR peaks. HR data was plotted
in a time/HR graph to ensure all peaks found by the
script matched the plotted peaks.
3. Determination of mean heart rate for all 3
segments.
4. Determination of HR amplitude and HRV for
all 3 segments.
5. Analysis of EDA time latency, response rise
time and readaption slope via plotting and linear
regression.
These steps were repeated for all 10 subjects.
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
236

3 RESULTS
Although it might be a difficult task to study the
influence of electrical stimulation due to external
factors, which are hard to control, the parameters
chosen provide a subjective analysis of the subjects’
reactions to stimulation. Parameters chosen for EDA
include EDA latency time, EDA rise time and EDA
readaptation slope.
Table 1 shows the HR and HRV for each subject
by determining standard deviation of the successive
hearth pulses intervals.
Table 1: Mean values and respective standard deviation
error for the heart rate (in beats per minute) obtained
during segments 1, 2 and 3.
Heart Rate (bpm)
Segment 1 Segment 2 Segment 3
Mean
(SD) Mean (SD) Mean (SD)
Subject
1
80.32 (8.79) 81.57 (8.24) 79.00 (8.26)
Subject
2
66.57 (7.99) 64.87 (5.91) 66.99 (8.24)
Subject
3
69.52 (2.89) 71.96 (3.81) 69.32 (3.25)
Subject
4
64.28 (5.57) 61.05 (4.15) 64.49 (5.44)
Subject
5
70.09 (5.62) 64.40 (4.88) 69.84 (5.38)
Subject
6
72.43 (5.68) 69.80 (3.72) 72.07 (4.66)
Subject
7
67.14 (4.40) 71.01 (6.43) 65.47 (4.85)
Subject
8
83.23 (5.53) 83.85 (5.86) 83.19 (5.97)
Subject
9
61.94 (6.32) 61.74 (5.01) 60.63 (6.36)
Subject
10
63.17 (4.22) 62.37 (3.22) 63.18 (3.68)
Figure 1 shows the subjects’ EDA during segment 1
and segment 2. Regarding the subject’s EDA from
segment 1 (Figure 1A), analysis of the results
showed that prior to the application of the stimuli
small oscillations were detected but deemed of low
importance. For segment 3, the behaviour was the
same as presented in Figure 1A. The EDA signals
from all subjects during segment 2 of the acquisition
protocol are presented in Figure 1B.
Table 2 presents EDA latency for all subjects and
for each burst of 20 stimuli. Negative values are
result of a response that was detected before the
burst was applied. There was no measurable
response from subjects 8 and 10 and subject 5
presented a signal with very low activity, which was
particularly hard to analyse.
Table 3 presents the EDA rise time for each of
the detected subjects’ EDA pulses to rise and peak.
4 DISCUSSION
The EDA signal in its absolute value has high inter-
subject variability, which makes it difficult to
establish a measurement range capable of
comprising significant population. To overcome this
effect, it is mandatory to establish amplitude / gain
independent objective parameters. In this study, only
7/10 subjects revealed significant responses of
sympathetic nervous system to the electrical
stimulation. For segment 1, EDA results showed, in
general, a continuous smooth descent tendency for
all subjects (Fig. 1A). For segment 2, EDA results
showed, for most of the subjects, that the EDA
signal has some events in response to the electric
stimulus (Fig. 1B). This allows the analysis of the
time latency between the reception of the stimulus
by the median nerve and the ANS reaction to it.
The stimulus/response latency is a parameter
inherent to the personal physiology and
consequently has high inter-subject variability. In
half of the subjects analysed, a slight tendency to
decrease the latency with the increase of the
stimulation intensity is noticed. This was expected
given the ANS constant re-adaptations.
One subject reveals a notorious response arousal
even before the stimulus application. Curiously, due
to poor quality of the acquisition for this subject, the
protocol needed to be repeated and the results here
exposed are from the second acquisition. This was
the only subject who had been exposed to the
protocol before.
When analysing the EDA rise time, a lower
standard deviation both intra and inter subject is
observed, when compared with Segment 1. The
response rise time seems to be a parameter which
does not correlate with the increase of stimulus
intensity, presenting very stable results within the
same subject.
Another pointer for the ANS management used
in this work was the analysis of BVP signal.
The BVP signal constitutes per se a direct signal
from the vascularization physiology. With the
cardiac pulse, erythrocytes will change the spatial
alignment and this will affect the blood’s opacity.
Multi-biosignalsAnalysis-TheEffectofPeripheralNerveStimulationonSkinConductanceandHeartRateVariability
237
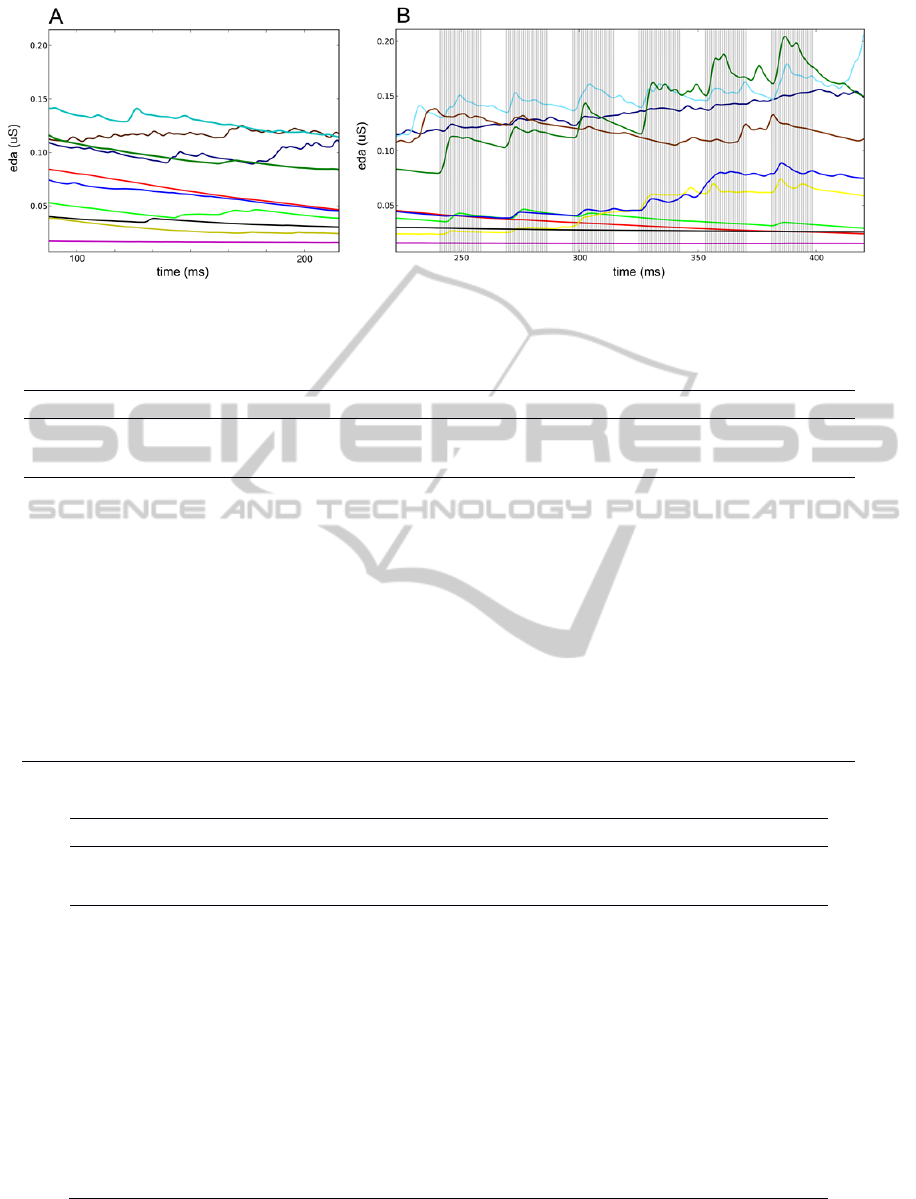
Figure 1: EDA signals obtained for all subjects. A) Example window obtained from segment 1 of the acquisition protocol
(basal activity); B) Signal obtained from segment 2 of the acquisition protocol (six bursts of electrical stimulation).
Table 2: Stimulus response latency of EDA signal for each stimulation burst.
Latency (s)
Burst 1
5mA
Burst 2
10mA
Burst 3
15mA
Burst 4
20mA
Burst 5
25mA
Burst 6
30mA
Mean
(SD)
Subject 1
2,05 1,60 1,30 0,92 2,34 1,19
1,57 (0,49)
Subject 2
0,00 0,00 0,95 1,00 0,88 0,89
0,63 (0,43)
Subject 3
-- -- -- -- -- --
--
Subject 4
0,92 0,83 0,80 1,14 1,16 0,73
0,93 (0,17)
Subject 5
-- -- -- -- -- --
--
Subject 6
2,42 2,16 1,55 0,59 1,02 0,97
1,45 (0,66)
Subject 7
-- * 1,20 4,40 -- 1,92 -- *
2,51 (1,37)
Subject 8
2,94 2,00 2,00 1,66 1,70 1,13
1,91 (0,55)
Subject 9
2,81 2,80 3,00 -- -- 0,99
2,40 (0,82)
Subject 10
-- -- -- -- -- -- --
*subject with event previous to stimulation
Table 3: Stimulus response rise time of EDA signal for each stimulation burst.
Rise Time (s)
Burst 1
5mA
Burst 2
10mA
Burst 3
15mA
Burst 4
20mA
Burst 5
25mA
Burst 6
30mA
Mean (SD)
Subject 1
2,74 4,00 3,70 3,00 2,36 3,94
3,29 (0,63)
Subject 2
4,74 4,19 4,55 4,92 3,47 4,54
4,40 (0,47)
Subject 3
-- -- -- -- -- --
--
Subject 4
7,63 3,13 6,40 3,28 2,93 5,60
4,83 (1,82)
Subject 5
-- -- -- -- -- --
--
Subject 6
3,14 3,15 3,93 5,03 2,50 2,83
3,43 (0,84)
Subject 7
-- * 3,10 2,59 -- 3,00 -- *
2,89 (0,22)
Subject 8
3,84 4,00 4,09 3,75 4,20 2,90
3,79 (0,43)
Subject 9
5,68 4,56 4,30 -- -- 3,93
4,62 (0,65)
Subject 10
-- -- -- -- -- -- --
*subject with event previous to stimulation
BIOSIGNALS2015-InternationalConferenceonBio-inspiredSystemsandSignalProcessing
238

The BVP signal of 5/10 subjects showed an
amplitude decreasing with the beginning of the
electrical stimulation. An example of this pattern can
be seen on the subject of Fig. 4. This is justified by a
vasoconstriction effect caused by adaptations of the
ANS to the electrical stimulation. Vasoconstriction
of the blood vessels increases the spatial density and
consequent alignment of erythrocytes. This fact will
increase the opacity of the finger, leading to lower
signal detection by the sensor used for the detection
of the BVP signal.
The BVP peaks also enable the analysis of the
HR and HRV through the computation of the
standard deviation (SD) of subsequent peaks latency.
Regarding the HR analysis it was observed that the
majority of the subjects (5/10) showed a decrease in
the HR and HRV during stimulation. In 2/10
subjects the opposite effect is verified: the HR and
HRV increase during the stimulation period. The
remainder subjects do not show a direct influence of
the stimulation stage.
For the majority of the subjects, it is
understandable that the electrical stimulation has an
influence on the ANS, which affects the vagus nerve
and thus the sinus node. For all subjects, on Segment
3, HR and HRV return to the Segment 1 basal
values. We can assume that after the stimulation the
subject’s ANS recovered easily. Therefore,
peripheral nerve stimulation did not have any short-
term consequences on the subjects’ HRV.
Electrical stimulation revealed to influence
several parameters of ANS. This influence can now
be taken into account on standard uses of peripheral
nerve stimulation.
REFERENCES
Visnovcova Z., Calvoska A. and Tonhajzerova I. (2013)
Heart rate variability and electrodermal activity as
noninvasive indices of sympathovagal balance in
response to stress, Comenius University, Slovak
Republic.
Thong, T., Li, K, Mcnames, J., Aboy, M., Goldstein, B.
(2003) Accuracy of Ultra-Short Heart Rate Variability
Measures, Oregon, United States of America.
Malik, M. (2006) Heart rate variability – Standards of
measurement, physiological interpretation and clinical
use, American Society for Pacing and
Electrophysiology.
Clifford, G., Tarassenko, L. (2002) Signal Processing
methods for Heart Rate Variability, Department of
Engineering Science, University of Oxford.
Dominik B., Guillaume F., Friston, K., Dolan, R. (2010),
Modulating event-related skin responses, University
College London, United Kingdom.
Malik, M., Camm, A. (1990) Heart rate variability –
Electrophysiology, pacing and arrhythmia, St.
George’s Hospital Medical School, London, England.
Araujo, T., Nunes, N., Gamboa, H. (2012) Miniaturized
wireless controlled electrostimulator. In proceedings
of biodevices – international conference on biomedical
electronics and devices. Biostec 2012, Vilamoura,
Portugal.
PLUX – Wireless Biosignals, SA (2014) [Online]
Available from http://www.plux.info [Accessed July
7
th
, 2014]
Multi-biosignalsAnalysis-TheEffectofPeripheralNerveStimulationonSkinConductanceandHeartRateVariability
239
