
On the Sol-gel Preparation of Selected Lanthanide Aluminium
Garnets Doped with Europium
L. Pavasaryte
1
, B. J. Lopez
2
and A. Kareiva
1
1
Department of Inorganic Chemistry, Vilnius University, Naugarduko 24, LT-03225 Vilnius, Lithuania
2
Departament de Química Inorgànica i Orgànica, Universitat Jaume I, E-12071 Castelló de la Plana, Spain
Keywords: Lanthanide Aluminium Garnets, Holmium, Terbium, Dysprosium, Doping, Europium, Sol-gel Processing,
XRD, FTIR, SEM, DLS, Fluorescence.
Abstract: A sol-gel method based on in-situ generation of mixed-metal chelates by complexing metal ions with
ethane-1,2-diol in an aqueous media has been elaborated to prepare lanthanide-ion containing garnets,
Tb
3
Al
5
O
12
(TAG), Dy
3
Al
5
O
12
(DAG) and Ho
3
Al
5
O
12
(HAG) doped with different amount of Eu. The X-ray
diffraction patterns (XRD) of the powders sintered at 1000 °C showed the formation of monophasic TAG,
DAG and HAG. The phase composition of the samples was also characterized by FTIR spectroscopy.
Microstructural features of the polycrystalline samples were studied by scanning electron microscopy
(SEM) and dynamic light scattered measurements (DLS). Luminescence properties were investigated by
laser and fluorescence spectrophotometer.
1 INTRODUCTION
The yttrium aluminium garnet (Y
3
Al
5
O
12
, YAG)
doped with a transition metal or lanthanide ions is an
important solid-state laser material widely used in
luminescence systems, window materials for a
variety of light sources, and for fiber-optic
telecommunication systems. The YAG oxides are
also widely applied as phosphors in cathode-ray
tubes (projection TV sets), field emission, vacuum
fluorescent, and electroluminescent displays and as
scintillators in X-ray and positron emission
tomographs (Harlan et al. 1997; Vaqueiro et al.
1998; Pullar et al. 1999; Ganschow et al. 1999; Kang
et al. 1999; Lu et al. 2000; Lu et al. 2002; Hreniak et
al. 2002; Pan et al. 2004; Potdevin et al. 2006; Singh
et al. 2007; Katelnikovas et al. 2007; Caponetti et al.
2007; Caponetti et al. 2007; Lipinska et al. 2007;
Katelnikovas et al. 2008; Khimich et al. 2009;
Suarez et al. 2009; Yang et al. 2009; Sun et al. 2009;
Fujioka et al. 2009; Yang et al. 2010). These
features have made rare-earth-doped YAG a relevant
material for cathode-ray tubes (CRTs), field
emission displays (FED), vacuum fluorescent and
electroluminescent displays, plasma display panel,
scintillators in X-ray and positron emission
tomographs and other luminescent applications.
The phosphors host materials have proven to be of
great importance for the optical function. The matrix
should possess good chemical, mechanical, thermal,
optical characteristics and properties. It is well
known that physical properties of crystalline
materials are very dependent on the host material,
phase purity, distribution of the grains sizes and
crystalline homogeneity. (Iida et al. 1999; Golubovic
et al. 2002; Zhang et al. 2008). For example,
transition metal and rare-earth element ions have
demonstrated lasing action in a wide variety of host
crystals. Among the compounds which can
incorporate transition metals or lanthanides several
scandium and gallium based materials were
elaborated (Vosegaard et al. 1997; Ferrand et al.
1999; Gaume et al. 2003; Muliuoliene et al. 2003;
Chenais et al. 2003; Garskaite et al. 2005; Mathur et
al. 2005; Sakirzanovas et al. 2008; Katelnikovas et
al. 2008). In the YAG all aluminium ions may be
substituted by gallium or iron ions, while pure
yttrium indium or yttrium scandium garnet is not
obtained. Besides, rare-earth aluminium garnets
have also attracted considerable attention as host
crystals for near-infrared solid-state lasers as well as
for optoelectronics devices, including computer
memories, microwave optical elements and as laser
active media with applications in medical surgery,
optical communications and coherent laser radar
(Papagelis et al. 2002; Papagelis et al. 2003;
Milanese et al. 2004).
Many different synthesis methods of synthetic
garnets are described in the literature. The solid-state
165
Pavasaryte L., Lopez B. and Kareiva A..
On the Sol-gel Preparation of Selected Lanthanide Aluminium Garnets Doped with Europium.
DOI: 10.5220/0005252901650171
In Proceedings of the 3rd International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS-2015), pages 165-171
ISBN: 978-989-758-093-2
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

reaction route is a widely used method for the
preparation of powders from a mixture of the solid
starting materials. Various wet-chemical methods,
which include combustion, co-precipitation,
hydrothermal, spray pyrolysis, sol-gel and emulsion
synthesis method have been developed and
successfully used for a low-temperature production
of phase-pure YAG, YGG, YIG powders and related
systems. Recently we have demonstrated that
monophasic yttrium aluminium garnet powders and
related garnet structure compounds with
homogeneously distributed lanthanide elements
within the garnet matrix can be successfully
synthesized by the simple aqueous sol-gel process.
The evaluated synthetic technique to garnet structure
compounds using acetate-nitrate-glycolate
intermediate illustrates the simplicity and superior
potential of the proposed method ( Katelnikovas et
al. 2007; Kareiva 2011; Skaudzius et al. 2014;
Zabiliute et al. 2014 ). The molecular level mixing
and the tendency of partially hydrolyzed species to
form extended networks facilitate the structure
evolution thereby lowering the crystallization
temperature. The reactivity of such precursors makes
the preparation of particular phases possible at
ambient and gentle conditions ( Livage et al. 1988;
Brinker et al. 1990; Cushing et al. 2004; Mackenzie
et al. 2007; Dubnikova et al. 2010 ).
Several lanthanide aluminium garnets (i.e.,
Tb
3
Al
5
O
12
, Dy
3
Al
5
O
12
and Ho
3
Al
5
O
12
) had not been
synthesized with various doping level of europium
using an aqueous sol-gel technique, to the best our
knowledge. Therefore, the main aim of this study
was to prepare Tb
3
Al
5
O
12
:Eu
3+
, Dy
3
Al
5
O
12
:Eu
3+
and
Ho
3
Al
5
O
12
:Eu
3+
using sol-gel technique and
investigate luminescent properties of these Eu
3+
-
doped garnets.
2 EXPERIMENTAL
The lanthanide aluminium garnet samples were
synthesized by an aqueous sol-gel method with
optimized synthesis parameters by mathematical
regression model (Katelnikovas et al. 2007;
Dubnikova et al. 2010 ). In the aqueous sol-gel
process, the following materials were used: Ho
2
O
3
(99.9 %), Tb
4
O
7
(99.9 %), Dy
2
O
3
(99.99%) and
Eu
2
O
3
(99.99%). Ho, Dy and Tb oxides were
dissolved in nitric acid (about 10 ml) with small
amount of water. Clear solutions were obtained after
stirring at 60-65 °C in beakers covered with a watch-
glass till dissolved. When oxides dissolved, mixture
washed with water till neutral pH. Then aluminium
nitrate nonahydrate and appropriate amount of
europium oxide dissolved in small amount of nitric
acid were added to above solutions and diluted till
100 ml. The resulting mixtures were stirred at 65 °C
for 1 h, followed by dropwice addition of ethane-
1,2-diol (HOCH
2
CH
2
OH) upon vigorous stirring.
The resulting sols were mixed at the same
temperature for another 1 h and then concentrated by
slow solvent evaporation at 65 °C until they turned
into transparent gels. The gels were dried in an oven
at 100 °C for 24 h. The resulting gel powders were
ground in an agate mortar and heated in air at 800 °C
for 4 h by slow temperature elevation (5 °C min
-1
).
After grinding in an agate mortar, the powders were
further sintered in air at 1000 °C temperature for
10h.
The synthesized samples were characterized by
X-ray powder diffraction (XRD) analysis, Fourier
transform infrared (FTIR) spectroscopy, scanning
electron microscopy (SEM), fluorescence
spectroscopy (FS) and dynamic light scattered
measurement (DLS). The XRD studies were
performed on D8 Bruker AXS powder
diffractometer using CuKα1 radiation. The
diffraction patterns were recorded at the standard
rate of 1.5 2θ / min. The FTIR spectra were recorded
with a Perkin-Elmer FT-IR Spectrum 1000
spectrometer. The scanning electron microscope
DSN 962 was used to study the surface morphology
and microstructure of the obtained ceramic samples.
Fluorescence data characterized by fluorescence
spectrophotometer Cary Eclipse Varian. Particle size
were identified by Zetasizer, nano series.
3 RESULTS AND DISCUSSION
3.1 XRD Characterization of LnAG:Eu
Powders
The phase purity and compositional changes of the
LnAG doped with different amount of Eu ions were
controlled by X-ray diffraction analysis. The XRD
patterns of corresponding Tb
3
Al
5
O
12
(TAG),
Dy
3
Al
5
O
12
(DAG) and Ho
3
Al
5
O
12
(HAG), with
different doping level of Eu powders annealed at
1000
o
C are shown in Figs. 1-3, respectively.
The XRD results presented in Fig. 1 revealed that
ceramics obtained at 1000 °C consists of one
crystalline phase: terbium aluminium garnet
(Tb
3
Al
5
O
12
, TAG). The obtained XRD patterns are
in a good agreement with the reference data for
Tb
3
Al
5
O
12
(PDF [04-006-4054]). Fig. 2 shows that
only one dysprosium aluminium garnet Dy
3
Al
5
O
12
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
166
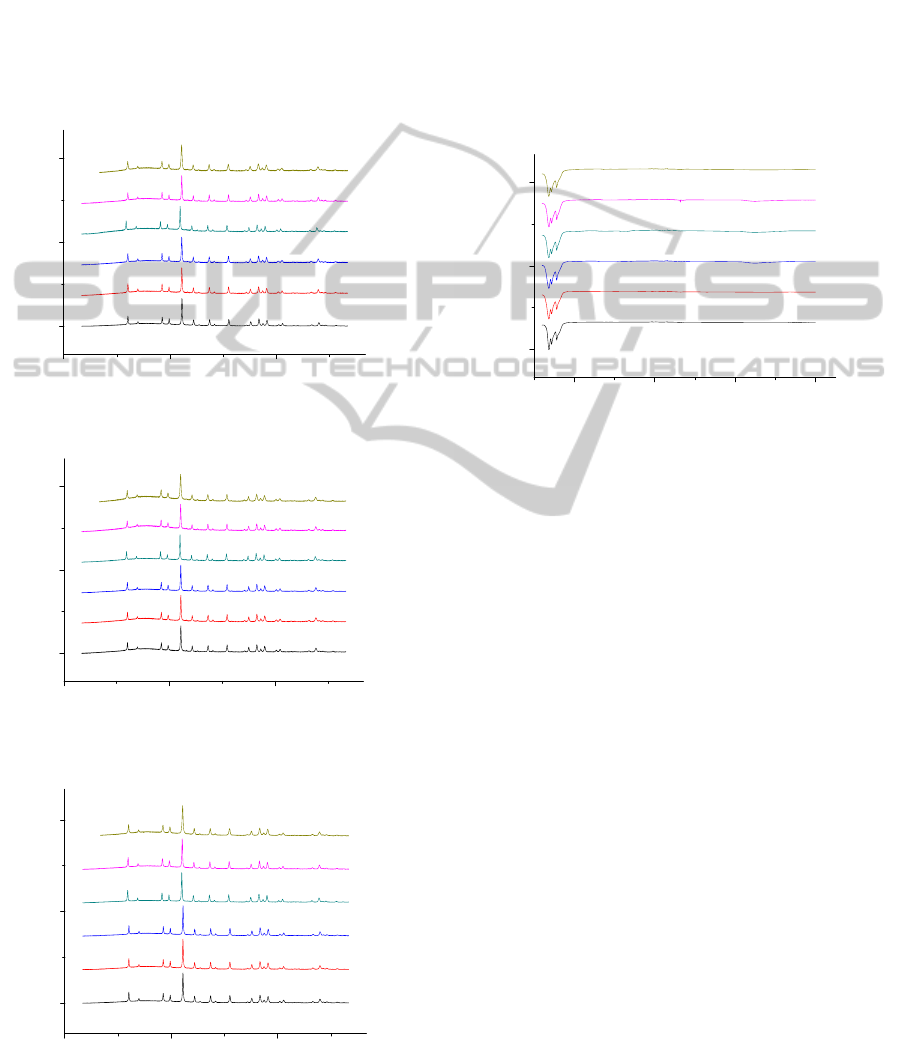
phase (PDF [04-006-4053]) was obtained after
calcination of Dy-Al-O:Eu precursors at 1000 °C. In
the case of the Ho-Al-O system, the analogous
results with of the Tb-Al-O and Dy-Al-O systems
were obtained. In Fig. 3 the X-ray diffraction
patterns of the final Ho-Al-O ceramic samples
annealed at 1000 °C are presented. The XRD
patterns prove the formation of the cubic holmium
aluminium garnets (Ho
3
Al
5
O
12
). Evidently, the
obtained XRD patterns are in a good agreement with
the reference data for Ho
3
Al
5
O
12
(PDF [04-001-
03060
0
3
6
0% Eu
0.1% Eu
0.25% Eu
0.5% Eu
0.75% Eu
1% Eu
Relative intensity
2
θ
Figure 1: XRD patterns of the Tb-Al-O:Eu gels annealed
at 1000
o
C.
03060
0
3
6
0% Eu
0.1% Eu
0.25% Eu
0.5% Eu
0.75% Eu
1% Eu
Relative intensity
2θ
Figure 2: XRD patterns of the Dy-Al-O:Eu gels annealed
at 1000
o
C.
03060
0
3
6
0% Eu
0.1% Eu
0.25% Eu
0.5% Eu
0.75% Eu
1% Eu
Relative intensity
2θ
Figure 3: XRD patterns of the Ho-Al-O:Eu gels annealed
at 1000
o
C.
9715]). Thus, we can conclude that the 1000
o
C
temperature is enough for the formation of Eu
3+
-
doped Tb
3
Al
5
O
12
, Dy
3
Al
5
O
12
and Ho
3
Al
5
O
12
garnets.
3.2 Infrared Spectroscopy
FTIR spectroscopy was used as additional tool for
the structural characterization of the ceramic
materials obtained by the aqueous sol-gel method.
The FTIR spectra of ceramic materials obtained after
the calcinations of the Ho-Al-O:Eu gels at 1000
o
C
for 10 h are shown in Fig. 4.
1000 2000 3000 4000
0
3
6
0% Eu
0.1% Eu
0.25% Eu
0.5% Eu
0.75% Eu
1% Eu
Transmittance %
Wavenumer [cm
-1
]
Figure 4: FTIR spectra of Ho
3
Al
5
O
12
:Eu
3+
garnets
synthesized at 1000
o
C.
The FTIR spectra of synthesized ceramics show
several quite intense broad bands at 675 cm
–1
, 720
cm
–1
and 775 cm
–1
,
which are typical metal-oxygen
(M-O) absorptions for the garnet-type compounds
(
Dubnikova et al. 2010; Garskaite et al. 2010; Li et al.
2005 ). The FTIR spectra of Tb-Al-O and Dy-Al-O
garnets doped with europium were very similar to
the presented in Fig. 4. The most important feature is
that intensive bands are determined in the region of
900–450 cm
-1
,
which may be also attributed to the
stretching modes of the isolated [AlO
4
] tetrahedra
and [AlO
6
] octahedra in the garnet structure, i.e.
these bands correspond to the formation of
crystalline TAG:Eu, DAG:Eu and HAG:Eu. Thus,
the observed M–O vibrations which in view of the
earlier reports are characteristic of RE–O and Al–O
stretching frequencies let us to conclude, that the
FTIR results are consistent with crystallization
process observed by XRD measurements.
Consequently, the FTIR results absolutely support
the conclusions made on grounds of the XRD
measurement and prove that there is no left organic
matter in the sol-gel derived garnet structure
compounds (
Li et al. 2005; Li et al. 2004; Xing et al.
2004; Pralad et al. 2013).
OntheSol-gelPreparationofSelectedLanthanideAluminiumGarnetsDopedwithEuropium
167

3.3 Scanning Electron Microscopy
The textural properties of the calcined Tb-Al-O:Eu,
Dy-Al-O:Eu, Ho-Al-O:Eu powders were
investigated by SEM, from which the grain size and
typical morphologies were obtained. Scanning
electron micrographs of the Tb-Al-O:0.5%Eu, Dy-
Al-O:0.5%Eu and Ho-Al-O:0.5%Eu samples
calcined at 1000
o
C are shown in Figs. 5-7,
respectively. The SEM once again proved to be a
valuable technique for the morphological
characterization of ceramic samples. Individual
particles seem to be submicro-sized plate-like
crystals and they partially
fused to form hard
agglomerates. The SEM micrograph presented in
Fig. 5 shows the formation of very homogeneous
mixed-metal oxide, and the formation of a
continuous network of crystallites is characteristic
feature for ceramic composite material during
calcination. The Ho-Al-O:Eu and Dy-Al-O:Eu
garnet particles are a little differently shaped
Figure 5: SEM micrograph of the Tb
3
Al
5
O
12
:0.5% Eu
3+
garnet.
Figure 6: SEM micrograph of the Dy
3
Al
5
O
12
:0.5% Eu
3+
garnet.
comparing with Tb-Al-O:Eu garnet (see Figs. 6 and
7). Apparently, the particles were formed with more
pronounced agglomeration, indicating good
connectivity between the grains which is
characteristic feature for ceramic composite material
(
Skaudzius et al. 2008).
Figure 7: SEM micrograph of the Ho
3
Al
5
O
12
:0.5% Eu
3+
garnet.
3.4 Dynamic Light Scattered
Measurements
The particle size distribution in Ho
3
Al
5
O
12
:Eu
garnets annealed at 1000 °C for 10 h were
investigated by dynamic light scattering (DLS). The
DLS measurements showed well size distribution of
garnets. When Eu doping level les or equal 0.1%
possible very narrow particle size distribution (~420
nm) was determined. The sample with higher
europium concentration (0.5%) were composed with
larger particles (~700 nm). When garnet doped with
0.25% - 0.75% of Eu
3+
some particles were much
more larger, and consequently the particle size is not
stable and split in a biggest range. However, the
biggest particle size was determined for the sample
with 1% of doping level of Eu. Interestingly, the
DLS results fit very well with scanning electron
microscopy data showing that 0.5% of Eu is the
optimum doping level for Tb, Dy and Ho aluminium
garnets having very homogeneous particle size
distribution.
3.5 Optical Properties
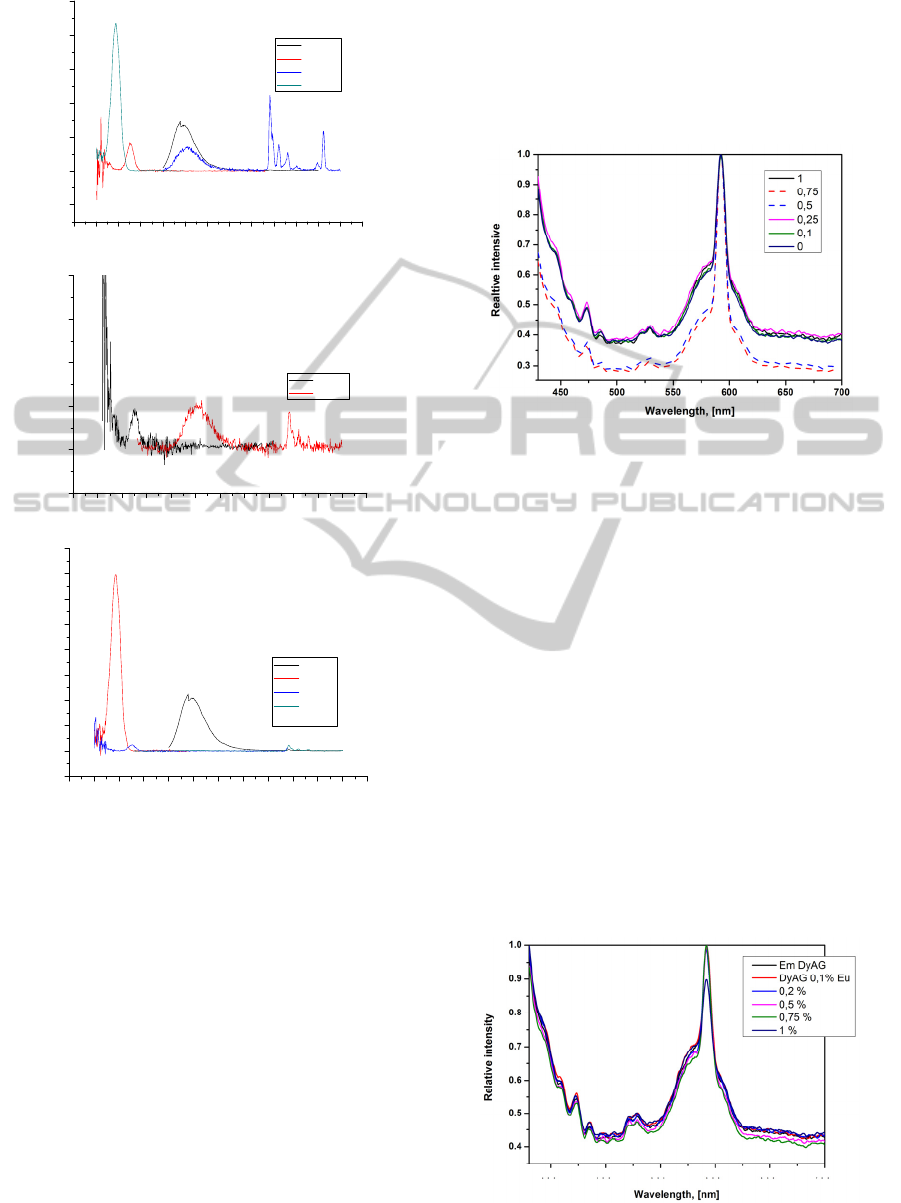
The fluorescence data of Ho
3
Al
5
O
12
:Eu are shown in
Fig. 8.
As seen. the 0.5% Eu-doped sample shows the
most intensive europium peaks between investigated
the Ho-Al-O:Eu garnet structure compounds.
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
168
150 200 250 300 350 400 450 500 550 600 650 700 750 800
-20
0
20
40
60
80
100
Intensity (a.u.)
Wavelenght (nm)
ex 242
em 591
ex275
em394
HoAG 0.5%Eu
150 200 250 300 350 400 450 500 550 600 650 700 750
-0.5
0.0
0.5
1.0
1.5
2.0
Intensity (a.u.)
Wavelength (nm)
em591
ex275
HoAG 0.75%Eu
150 200 250 300 350 400 450 500 550 600 650 700 750
-5
0
5
10
15
20
25
30
35
40
Intensity (a.u.)
Wavelenght (nm)
ex242
em394
em591
ex275
HoAG 1%Eu
Figure 8: Fluorescence measurement data of
Ho
3
Al
5
O
12
:Eu
3+
garnets.
This data fit well with XRD results (peaks with
0,5% europium are shifted to the smaller 2θ). The
sample having 1% of Eu shows possible
fluorescence, however, not so intensive as with 0.5%
of Eu, but more intensive than 0.75% Eu-doped
sample. The XRD data also showed the smaller shift
for 0.75% Eu and 1% Eu samples. Finally, the
results of fluorescence measurement support the
proposition that the best doping level for HAG:Eu is
0.5% of europium.
It is particularly well known that, phosphors with
spherical shaped particles (≤ 2 µm) are of greater
importance because of their high packing density,
lower scattering of light, brighter luminescent
performance, high definition and more improved
screen packing density. This explain why 0.5
europium doping shows the best luminescent
properties (
Raju et al. 2008; Park et al. 2010).
Figs. 9 and 10 represent luminescence spectra of
Tb
3
Al
5
O
12
:Eu and Dy
3
Al
5
O
12
:Eu garnets,
respectively.
Figure 9: Luminescent (emission) spectra of
Tb
3
Al
5
O
12
:Eu
3+
garnets.
The spectra were acquired by using 394 nm and
275 nm excitation. The emission peaks were fixed
between 550 and 650 nm, and well agree with the
reported values of Eu
3+
emission transitions [56-58].
The emission spectral lines of Eu
3+
ion are sharp
which is due to the screening of 4f orbital by 5s and
5p orbitals from crystal field of the host lattice.
Spectrums show the characteristic emission of Eu
3+
arising due to
5
D
0
→
7
F
1
(591nm),
5
D
0
→
7
F
2
(611nm) and
5
D
0
→
7
F
4
(708nm) transitions (Singh et
al. 2013). As expected for the Eu
3+
ions a typical
strong red emission was present with the most
intense line at 611 nm originated from the
5
D
0
→
7
F
2
hypersensitive transition (Redenka et al. 2014). Few of
Ho
3
Al
5
O
12
:Eu doping (0.1% Eu, 0.25% Eu) do not
show any emission peaks. The results obtained are in
a good agreement with fluorescence data, beside
with this doping level peaks in XRD graphs did not
shift.
Figure 10: Luminescent (emission) spectra of
Dy
3
Al
5
O
12
:Eu
3+
garnets.
OntheSol-gelPreparationofSelectedLanthanideAluminiumGarnetsDopedwithEuropium
169

Fig. 11: represents the life time of Ho
3
Al
5
O
12
:Eu
garnets obtained after annealing at 1000 °C for 10 h.
0 5 10 15 20
5
7
6
7
ln(I) [a.u.]
Time [ms]
0,1% Eu
0,25% Eu
0,5% Eu
0,75% Eu
1% Eu
5
Figure 11: The life time measurements for the
Ho
3
Al
5
O
12
:Eu
3+
garnets.
The luminescence lifetime for the most intense
emission line at 611 nm of Eu
3+
(
5
D
0
→
7
F
1
) were
recorded using 275 nm as excitation source. The
obtained photoluminescence lifetimes show a
decrease (Fig. 12) from 2.58 to 1.98 ms with
increasing doping concentration. Upon increasing
the doping concentration, the decay becomes faster.
In our case the maximum decay time is 2.58 ms with
0,1 mol% doping concentration. Increasing doping
content may increase the number of ions occupying
the surface states in nanoscale dimensional materials
and these states may be leading to concentration –
quenching behaviour (
Packiyaraj et al. 2014;
Muenchausen et al. 2007)
. Decay time decrease with
increasing doping content. This result supports
emission and excitation spectral analysis.
0,0 0,2 0,4 0,6 0,8 1,0
1,8
1,9
2,0
2,1
2,2
2,3
2,4
2,5
2,6
2,7
Lifetime (ms)
Eu Concentration (%)
Figure 12: The decay curves for Ho
3
Al
5
O
12
:Eu
3+
garnet.
4 CONCLUSIONS
The sinterability and microstructal evolution of
synthesized lanthanide aluminium garnets by an
aqueous sol-gel process were investigated in the
present study. In this work aqueous sol-gel process
was used to prepare lanthanide-aluminium oxides
Tb
3
Al
5
O
12
,
Dy
3
Al
5
O
12
and Ho
3
Al
5
O
12
doped with
different amount of europium. It was concluded
from the XRD data that monophasic Tb
3
Al
5
O
12
:Eu,
Dy
3
Al
5
O
12
:Eu
and Ho
3
Al
5
O
12
:Eu
garnets can be
easily synthesized at 1000 °C using the proposed
sol-gel chemistry approach. Europium inside garnet
structure slightly shifted the reflection peaks in the
XRD patterns. The biggest shift was observed in the
XRD pattern of the samples with 0.5% of Eu. The
XRD results were supported by FTIR, SEM, DLS
fluorescence and luminescent measurements. It was
also determined that the amount of europium inside
garnet structure influenced the particle size. With
increasing doping level the particle size also
increased. The luminescence lifetime for the most
intense emission line at 611 nm of Eu
3+
(
5
D
0
→
7
F
1
)
was recorded using 275 nm as excitation source in
HAG and 394 nm in TAG and DAG. Upon
increasing the doping concentration, the decay
became faster in Ho
3
Al
5
O
12
:Eu. The characterization
of synthesized samples using all measurements used
in this study resulted to the conclusion that that 0.5%
of europium is optimum doping level. Thus, the
developed synthesis route offers unique
opportunities for the synthesis of optical materials,
since it is suited for the production of thin/thick
films, monoliths and fibbers.
REFERENCES
C. J. Harlan, A. Kareiva, D. B. MacQueen, R. Cook, and
A. R. Barron, 1997. Adv. Mater. 9, 68.
P. Vaqueiro, and M. A. Lopez-Quintela, 1998. J. Mater.
Chem. 8, 161 (1998).
R. C. Pullar, M. D. Taylor, and A. K. Bhattacharya, 1999.
J. Eur. Ceram. Soc. 19, 1747.
S. Ganschow, D. Klimm, P. Reiche, and R. Uecker, 1999.
Cryst. Res. Technol. 34, 615.
Y. C. Kang, I. W. Lenggoro, S. B. Park, and K. Okuyama,
1999. J. Phys. Chem. Solids 60, 1855.
J. Lu, M. Prabhu, J. Song, C. Li, J. Xu, K. Ueda, A. A.
Kaminskii, H. Yagi, and T. Yanagitani, 2000. Appl.
Phys. B 71, 469.
C. H. Lu, H. C. Hong, and R. Jagannathan, 2002. J. Mater.
Chem. 12, 2525.
D. Hreniak, and W. Strek, 2002. J. All. Comp. 341, 183.
Y. Zhou, J. Lin, M. Yu, S. Wang, and H. Zhang, 2002.
Mater. Lett. 56, 628.
Y. Pan, M. Wu, and Q. Su, 2004. J. Phys. Chem. Solids
65, 845.
A. Potdevin, G. Chadeyron, D. Boyer, and R. Mahiou,
2006. J. Sol-Gel Sci. Techn. 39, 275.
R. Singh, R. K. Khardekar, A. Kumar, and D. K. Kohli,
2007. Mater. Lett. 61, 921.
PHOTOPTICS2015-InternationalConferenceonPhotonics,OpticsandLaserTechnology
170

A. Katelnikovas, P. Vitta, P. Pobedinskas, G. Tamulaitis,
A. Zukauskas, J.-E. Jørgensen, and A. Kareiva, 2007.
J. Cryst. Growth 304, 361.
E. Caponetti, M. L. Saladino, F. Serra, and S. Enzo, 2007.
J. Mater. Sci. 42, 4418.
E. Caponetti, S. Enzo, B. Lasio, and M. L. Saladino, 2007
Opt. Mater. 29, 1240.
L. Lipinska, L. Lojko, A. Klos, S. Ganschow, R.
Diduszko, W. Ryba-Romanowski, and A.
Pajaczkowska, 2007. J. All. Comp. 432, 177.
A. Katelnikovas, T. Justel, D. Uhlich, J.-E. Jorgensen, S.
Sakirzanovas, and A. Kareiva. 2008. Chem. Eng.
Comm. 195, 758.
N. N. Khimich, E. N. Poddenezhnyi, A. A. Boiko, A. V.
Zdravkov, V. L. Ugolkov, L. A. Koptelova, E. I.
Grishkova, and A. O. Dobrodei, 2009. Glass Phys.
Chem. 35, 504.
M. Suarez, A. Fernandez, J. L. Menendez, and R.
Torrecillas, 2009. J. Nanomater. Art. # 138490.
H. J. Yang, L. Yuan, G. S. Zhu, A. B. Yu, and H. R. Xu,
2009. Mater. Lett. 63, 2271.
X. X. Ge, Y. H. Sun, C. Liu, and W. K. Qi, 2009. J. Sol-
Gel Sci. Technol. 52, 179.
K. Fujioka, T. Saiki, S. Motokoshi, Y. Fujimoto, H. Fujita,
and M. Nakatsuka, 2009. Ceram. Int. 35, 2393.
H. K. Yang, and J. H. Jeong, 2010. J. Phys. Chem. C 114,
226.
Y. Iida, A. Towata, T. Tsugoshi, and M.Furukawa, 1999.
Vibr. Spectr. 19, 399.
A. Golubovic, S. Nikolic, R. Gajic, S. Duric, and A.
Valcic, 2002. J. Serb. Chem. Soc. 67, 291.
L. Zhang, C. Y. Zhang, D. H. Li, Z. Y. Wei, Z. G. Zhang,
J. E. Hans, and S. Strohmaier, 2008. Chin. Phys. Lett.
25, 3988.
T. Vosegaard, D. Massiot, N. Gautier, and H. J. Jakobsen,
1997. Inorg. Chem. 36, 2446.
B. Ferrand, B. Chambaz, and M. Couchaud, 1999. Opt.
Mater. 11, 101.
R. Gaume, B. Viana, J. Derouet, and D. Vivien, 2003. Opt.
Mater. 22, 107.
I. Muliuoliene, S. Mathur, D. Jasaitis, H. Shen, V.
Sivakov, R. Rapalaviciute, A. Beganskiene, and A.
Kareiva, 2003. Opt. Mater. 22, 241.
S. Chenais, F. Druon, F. Balembois, P. Georges, A.
Brenier, and G. Boulon, 2003. Opt. Mater. 22 99.
E. Garskaite, Z. Moravec, J. Pinkas, S. Mathur, R.
Kazlauskas, and A. Kareiva, 2005. Phil. Magaz. Lett.
85, 557.
S. Mathur, H. Shen, A. Leleckaite, A. Beganskiene, and A.
Kareiva, 2005. Mater. Res. Bull. 40, 439.
S. Sakirzanovas, L. Sun, Ch. Yan, and A. Kareiva, 2008.
Mendeleev Commun. 18, 251.
A. Katelnikovas, and A. Kareiva. 2008. Mater. Lett. 62,
1655.
K. Papagelis, G. Kanellis, T. Zorba, S. Ves, and G. A.
Kourouklis, 2002. J. Phys. Condens. Matter 14, 915.
K. Papagelis, and S. Ves, 2003. J. Phys. Chem. Solids 64,
599.
C. Milanese, V. Buscaglia, F. Maglia, and U. Anselmo-
Tamburini, 2004. Chem. Mater. 16, 1232.
A. Katelnikovas, J. Barkauskas, F. Ivanauskas, A.
Beganskiene, and A. Kareiva, 2007. J. Sol-Gel Sci.
Techn. 41, 193.
A. Kareiva, 2011. Materials Science (Medžiagotyra), 17,
428.
R. Skaudzius, A. Katelnikovas, D. Enseling, A. Kareiva,
and T. Juestel, J. Lumin. 147, 290 (2014).
A. Zabiliute, S. Butkute, A. Zukauskas, P. Vitta, and A.
Kareiva, 2014. Appl. Optics, 53, 907.
J. Livage, M. Henry, C. Sanchez, 1988. Progr. Solid State
Chem. 18, 259.
C. J. Brinker, G. W. Scherrer, 1990. Sol-Gel Science: The
Physics and Chemistry of Sol-Gel Processing,
Academic Press, San Diego.
B. L. Cushing, V. L. Kolesnichenko, and C. J. O‘Connor,
2004. Chem. Rev. 104, 3893.
J. D. Mackenzie, and E. P. Bescher, 2007. Acc. Chem.
Res. 40, 810.
N. Dubnikova, E. Garskaite, J. Pinkas, P. Bezdicka, A.
Beganskiene, A. Kareiva, 2010. J. Sol-Gel Sci. Techn.
55 (2), p.213 – 219.
E. Garskaite, N. Dubnikova, A. Katelnikovas, J. Pinkas,
and A. Kareiva, 2010. Collect. Czech. Chem.
Commun. 72, 321.
Xia Li, Hong Liu, Jiyang Wang, Hongmei Cui, Shunliang
Yang, I.R. Boughton, 2005. J. Phys. Chem. Solids 66
201-205.
Xia Li, Hong Liu, Jiyang Wang, Hongmei Cui, Feng Han,
2004. J. Am. Ceram. Soc., 87 [12] 2288-2290.
Lin Xing, Luming Peng, Min Gu, Guodong Tang, 2004. J.
Alloys Comp. 491 599-604.
Nathalie Pralad, Genevieve Chadeyron, Audrey Potdevin,
Jerome Deschamps, Rachid Mahiou, 2013. J. Eur.
Ceram. Soc. 33 1935-1945.
R. Skaudzius, A. Zalga, and A. Kareiva, 2008. Materials
Science - Medziagotyra 14, 193.
G.Seeta Rama Raju, S. Buddhudu, 2008. Mater. Lett. 62
1259.
Jin Young Park, Hong Chae Jung, G. Seeta Rama Raju,
Byung Kee Moon, Jung Hyun Jeong, Jung Hwan Kim.
2010. J. Lumin. 130 478 – 482.
R. Srinivasan, R. Yogamalar, A. Chandra Bose, 2010.
Meter. Res Bull. 45.
J. Dhanaraj, R. Jagannathan, T.R.N. Kutty, C.-Hsin Lu,
2001. J. Phys. Chem. B 105 11098.
P.Packiyaraj, P. Thangadurai, 2014. J. Lumin 145 997-
1003.
S. K. Singh, Dong Gi Lee, Soung Soo Yi, Kiwan Jang,
Dong-Soo Shi, Jung Hyun Jeong, 2013. J. Appl. Phys.
113, 173504.
Redenka M., Krsmanovič Whiffen, Željka Antic, Adolfo
Speghini, Mikhail G. Brik, Barbora Bartova, Marco
Bettinelli, Miroslav D. Dramicanin, 2014. Opt. Mater.
36 1083-1091.
P.Packiyaraj, P. Thangadurai, 2014. J. Lumin 145 997-
1003.
R. E.Muenchausen, L. G. Jacobsohn, B. L. Bennett, E. A.
McKigney, 2007. J. F. Smith, J. A. Valdez, D. W.
Cooke, J. Lumin, 126 838.
OntheSol-gelPreparationofSelectedLanthanideAluminiumGarnetsDopedwithEuropium
171
