
In Silico Analysis of Interactions Between NFkB and HSF Pathways
Jaroslaw Smieja
1
, Malgorzata Kardynska
1
, Anna Naumowicz
1
, Patryk Janus
1,2
,
Piotr Widlak
2
and Marek Kimmel
1,3
1
Institute of Automatic Control, Silesian University of Technology, Akademicka 16, 44-100 Gliwice, Poland
2
Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice 44–100, Poland
3
Department of Statistics, Rice University, Houston, TX 77005, U.S.A.
Keywords: Signalling Pathways, NFkB, HSF, HSP, Heat Shock.
Abstract: Motivation: Inhibition of NFkB pathway is known to promote apoptosis and therefore may constitute one of
the goals in anticancer therapies. Experimental results show that heat shock induces such inhibition in
cancer cells. However, the mechanisms of interactions between heat shock and NFkB pathways are not fully
understood yet. Development of a combined mathematical model of these pathways and its subsequent
computational analysis should help to uncover these mechanisms and determine the time window in which
heat shock treatment preceding chemotherapy would be the most efficient.
Results: An original mathematical model has been developed, allowing for computational testing of various
hypotheses concerning main sources of interplay between HSF and NFkB pathways. Computational
analysis strongly suggests that the competition for IKK, known from literature, cannot be the only
mechanism. Two plausible hypotheses are that either a kinase activating IKK can misfold due to heat shock
or that heat shock affects TNF receptors, blocking activation of NFkB pathway at the cell membrane.
1 INTRODUCTION
NFkB transcription factor regulates expression of
various genes, including those important for cell
survival. Therefore it has been the subject of
research for many years now, and its role in
promoting or blocking apoptotic pathways has been
investigated as one of the key molecular players
determining fate of cancer cells after radio- or
chemotherapy. In particular, various ways of
inhibition of NFkB pathway have been the focus of
attention in many cancer studies (e.g. Yamamoto
and Gaynor, 2001, Amman et al., 2009, Domingo-
Domènech et al., 2008, Zanotto-Filho et al., 2011).
On the other hand, hyperthermia was suggested
to have a potential of improving the efficacy of
chemo- or radiotherapy by many authors (e.g.
Neznanov et al., 2011) and be a promising treatment
in itself (Lee Titsworth et al., 2014). Bringing cells
under a heat stress initiates many biochemical
processes, including, among others, an apparent
inhibition of the NFkB pathway (Janus et al., 2011).
While the precise mechanism of this inhibition is not
clear, it has been observed that this inhibition
remains in place for some time after the source of
heat shock have been removed. Moreover, it is
known that HSF1 is the primary transcription factor
activated under stress conditions that is responsible
for induction of genes encoding heat shock proteins
(Fujimoto and Nakai, 2010, Morimoto 2011,
Westerheide et al., 2012), hence the HSF1 pathway
is a natural candidate for investigation. Uncovering
the mechanisms behind this inhibition should yield
two direct benefits: (i) finding the best (in terms of
maximum reduction of cancer cell population) time
lag between short thermal shock and subsequent
irradiation or chemotherapeutic agent delivery, (ii)
expanding knowledge about other possible
molecular interactions that could be utilized to
increase treatment efficacy even further.
Of the two components that are combined in this
work, the NFkB pathway has been studied much
more extensively and many models have been
developed so far. It appears that one of the most
often cited models is the one published in (Lipniacki
et al., 2004), whose structure was later modified to
some extent in later works (e.g., Wang et al., 2011,
Wang et al., 2012, Zambrano et al., 2014). Much
less attention was devoted to the HSF pathway,
though one can find several papers dealing with
201
Smieja J., Kardynska M., Naumowicz A., Janus P., Widlak P. and Kimmel M..
In Silico Analysis of Interactions Between NFkB and HSF Pathways.
DOI: 10.5220/0005256602010206
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2015), pages 201-206
ISBN: 978-989-758-070-3
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

mathematical modeling of the intracellular processes
that are initiated by heat shock (Rieger et al., 2005,
Szymanska and Zylicz, 2009, Petre et al., 2011,
Rybinski et al., 2013). To our knowledge, there has
been no published attempt to combine models of
both pathways into a single one, which could bring
some important conclusions about possible
inhibition of NFkB pathway by heat shock, and its
implications for clinical applications. While recently
some work has been done on analysis of NFkB
pathway dynamics under heat shock conditions, it
was done only through manipulating the parameters
of NFkB model (Sheppard et al., 2014). While such
approach might be welcome as it does not
necessitate increasing the complexity of the model,
it is not useful if the question posed concerns the
nature of interactions between the pathways, their
dynamics and possible crosstalk with other
pathways. In this work, we aim at building a model
that might help answering these questions.
2 METHODS
The model presented in this work is clearly
deterministic. While it has been proven that
stochasticity plays a crucial role in intracellular
processes described by signaling pathways models,
deterministic approach is much more convenient
when the initial model is developed. Stochastic
modeling should inevitably follow, in particular
when the analysis would be focused on determining
the ultimate fate of cells. That, however, is beyond
the scope of this paper.
Taking into account that the separate models of
each of the two pathways under consideration have
already been published, we decided to arbitrarily
chose their representatives and introduce the
necessary modifications concerning both the
proposed crosstalk mechanisms and parameter
values that would compensate the crosstalk in
control (i.e. not excited) cells. Additionally, the HSF
pathway has been further modified, to take into
account both assumptions needed to apply the law of
mass action that is behind the most part of the
deterministic modeling and the implications of
experimental results that are available.
The detailed description of canonical NFkB and
HSF pathways can be found in many papers,
including those cited in the Introduction section.
Here they will be only briefly summarized in the
following two paragraphs.
In unstimulated cells, NFkB forms a cytoplasmic
complex with its inhibitor IκB proteins. The
pathway can be activated, among others, by Tumor
Necrosis Factor (TNF) stimulation. Then, the kinase
IKK is activated and it subsequently phosphorylates
the inhibitors, targeting them for degradation. Freed
NFkB is imported into the nucleus, where it serves
as a transcription factor for many genes. These genes
include, among others, the genes coding IκB
inhibitors. Thus a negative feedback is formed.
Another feedback loop involves the A20 protein,
whose gene is also activated in the pathway.
In the heat shock activated pathway, in turn, the
main players are HSP and HSF proteins. Under
normal conditions, they mostly reside in complexes
with each other. Following heat shock, some of the
proteins in a cell denature and become misfolded.
These denaturated proteins cause HSP|HSF complex
to dissociate, following HSP binding to the
misfolded proteins. Free HSF molecules undergo
phosphorylation and subsequently form trimers,
which are transported into nucleus. Once there, the
trimers serve as transcription factors of heat-shock
inducible genes, including the family of HSP
proteins. Newly produced HSP proteins can bind
HSF, thus creating a negative feedback loop.
In all simulations aimed at checking the possible
inhibition of the NFkB pathway, the excitation
protocol comprised of a short, 30 minute heat shock
of 42 degrees, followed by TNF stimulation. The
time lag between the end of heat shock and
beginning of TNF treatment was varied, to check
when the NFkB pathway regains its functionality.
Cytoplasmic IKK and nuclear NFkB proteins were
chosen to represent the pathway response. In order
to check if the pathway was inhibited, the results
were compared to the ones obtained when only TNF
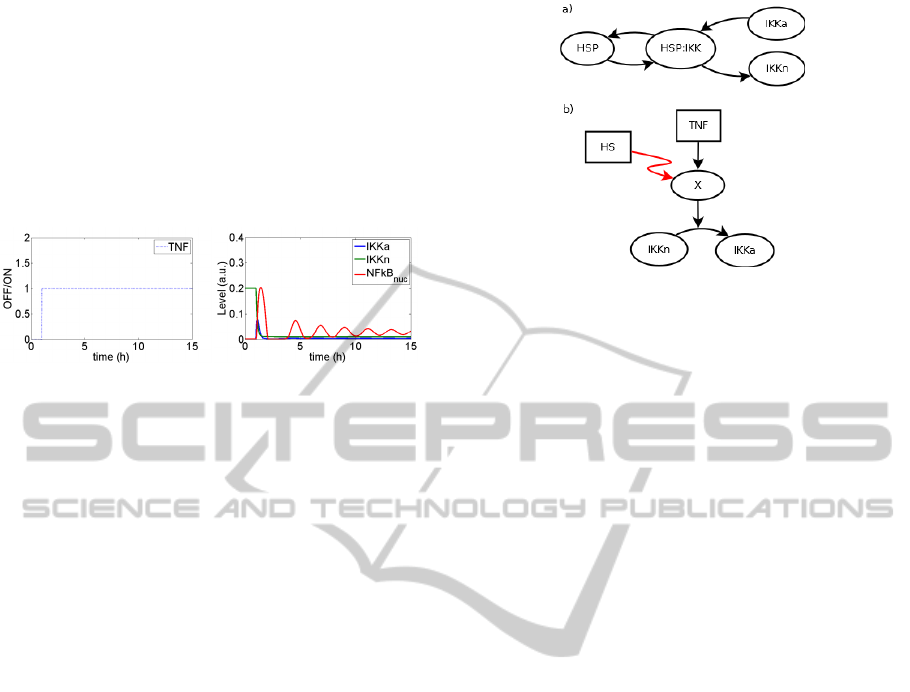
was used (see Figure 1).
It was assumed that the model should satisfy the
following assumptions, indicated by experimental
data (Ciocca and Calderwood 2005; Daugaard et al.,
2007, Morimoto, 2011, Janus et al., 2011 and our
own, unpublished results):
there are two types of HSP: constitutive, present
at high levels all the time, and inducible; though
they are represented by separate variables in the
model, they perform the same actions; therefore
the model should be applicable both in the case
when they are the same and when they are
different species of HSP.
Most of the constitutive HSP is located in the
cytoplasm in a normal physiological state.
Following the beginning of a heat shock, most of
the HSP is transported to the nucleus so that in
less than 1 hour most of it appears in the nucleus.
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
202
3 to 4 hours after the heat shock begun, inducible
HSP is observed in cytoplasm.
Of all possible complexes formed by HSF1 taken
into account in the model, only the trimer can
shuttle between nucleus and cytoplasm.
If the TNF is given earlier than 6 hours after heat
shock has ended, a partial inhibition of the NFkB
pathway is observed. If this time lag is greater
than 6 hours, inhibition does not take place.
Figure 1: The reference plots for not inhibited NFkB
pathway.
3 THE MATHEMATICAL MODEL
One of the most frequently mentioned mechanisms
of possible crosstalk between the two pathways
under consideration is the competition for IKK
proteins through creation of IKK|HSP complexes
(e.g. Ran et al., 2004). Therefore, this process has
been incorporated into our model and it involves
both constitutive and inducible HSPs (Figure 2a).
Another possible mechanism might involve a kinase
responsible for IKK activation. Heat shock causes,
among others, misfolding of some proteins. If that
kinase was misfolded (Figure 2b), it would be
unable to activate IKK (however, this could be
repaired by the constitutive HSP). Yet another
possibility is the effect of heat shock on the TNF
receptor. Receptor-dependent sensitivity of NF-κB
pathway has already been reported in another
context (Yang and Zhou, 2013). If TNF receptors
are inactivated by heat shock, then obviously the
NFkB pathway would be inhibited. In that case, one
could always choose model parameters to adjust the
time of inactivation and since the results of such
simulation are obvious, they are not presented in the
paper.
As indicated in the preceding section, the model
proposed in this work is built on the previously
published ones, which described either NFkB or
HSF1 pathways separately. Of these, the NFkB
model comes from (Lipniacki et al., 2004) and was
changed only in the parts that are responsible for the
crosstalk between the pathways, shown in the Figure
2 and summarized in the Table 1.
Figure 2: Possible crosstalk mechanisms between HSF and
NFkB pathways: (a) creation of IKK|HSP complexes, (b)
misfolding of kinase activating IKK.
The existing HSF1 pathway model (Szymanska
and Zylicz, 2009) had to be modified to a much
greater extent and these changes are summarized
below:
First, both this and other models (e.g. Rieger et
al., 2005, Petri et al., 2011) treat the cell as a
single compartment. Here, in order to satisfy the
assumptions presented in the preceding section,
it was necessary to distinguish cytoplasmic and
nuclear levels of proteins and complexes.
Constitutive and inducible HSPs are described by
separate variables.
The complexes of the HSF1 trimer and its
corresponding Heat Shock Element (HSE) in the
promoter region of the HSP gene are not
modeled explicitly. It seems that here law of
mass action is not applicable due to a small
number of HSEs and to take into account these
complexes properly one should use a stochastic
approach. Instead, transcription rate of the HSP
gene is assumed to be proportional to the nuclear
level of the trimer.
The function describing temperature-dependent
protein degradation rate has been modified from
its original version (Peper et al., 1997) which
was subsequently used in (Rybinski et al., 2013)
to be zero for the temperature of 37 degrees.
In addition to forming complexes with HSF1 and
misfolded proteins, HSP can also form
complexes with IKK.
While the introduction of nuclear and
cytoplasmic compartments, as well as constitutive
and inducible forms of HSP have not changed the
main reasoning behind the previously published
HSF pathway models, the number of changes might
be confusing without a proper presentation.
Therefore, the list of reactions in the model is
presented in the Table 1.
InSilicoAnalysisofInteractionsBetweenNFkBandHSFPathways
203
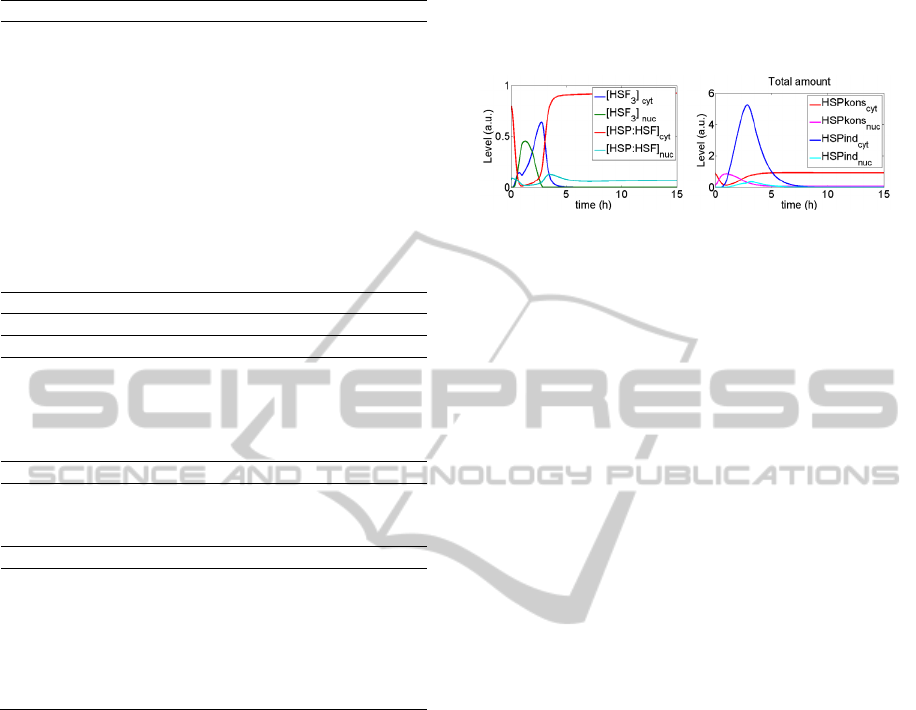
Table 1: Reaction list for the HSF pathway.
Reactions both in cytoplasm and nucleus:
Prot → mfProt
mfProt + HSP
cons
→ HSP
cons
:mfProt
mfProt + HSP
ind
→ HSP
ind
:Prot
HSP
cons
:mfProt → HSP
cons
+ Prot
HSP
ind
:Prot → HSP
ind
+ Prot
HSP
cons
+ HSF → HSP
cons
:HSF
HSP
ind
+ HSF ↔ HSP
ind
:HSF
HSP
cons
:HSF + mfProt → HSP
cons
:mfProt + HSF
HSP
ind
:HSF + mfProt → HSP
ind
:mfProt + HSF
3HSF → HSF
3
HSF3 + HSP
ind
→ HSP
ind
:HSF + 2 HSF
HSP
ind
→
mRNA →
Reactions taking place only in the nucleus:
HSF
3
→ HSF
3
+ mRNA
Reactions taking place only in cytoplasm:
mRNA → mRNA + HSPF
ind
HSF
cons
+ IKKa → HSP
cons
:IKK
HSF
ind
+ IKKa → HSP
ind
:IKK
HSP
cons
:IKK → HSP
cons
+ IKKn
HSP
in
d
:IKK → HSP
in
d
+ IKKn
Transport:
HSP
cons,cyt
↔ HSP
cons,nuc
HSP
ind,cyt
↔ HSP
ind,nuc
HSF
3 c
y
t
↔ HSF
3 nuc
Additional reactions in the model B
IKKn
,
IKKa
X
→ mfX
mfX + HSP
kons
→ HSP
kons
:mfX
mfX + HSP
ind
→ HSP
ind
:mfX
HSP
kons
:mfX → HSP
kons
+ X
HSP
in
d
:mfX → HSP
in
d
+ X
In all, three models have been tested. The first
one took into account IKK|HSP formation as the
only mechanism behind the inhibition of NFkB
pathway. Since it has yield no positive results, its
simulations are not included in the subsequent
section. The second model (Model A) additionally
incorporated nuclear import of HSP proteins as a
temperature-dependent process to reflect nuclear
accumulation of constitutive HSP following the heat
shock. In the third model (Model B), concentration
of a kinase activating IKK is explicitly modeled,
with the kinase being prone to misfolding in a
temperature-dependent manner.
4 RESULTS AND DISCUSSION
Due to abundance of constitutive HSP, despite
formation of HSP|IKK complexes the NFkB
pathway does not affect dynamics of the HSF1
pathway. Therefore, only exemplary results,
showing the main molecules in the HSF1 pathway
are shown in Figure 3. The remaining plots
concentrate on possible inhibition of the NFkB
pathway.
Figure 3: HSF and HSP levels following 30-minute heat
shock.
The initial assumption that the IKK|HSP
complexes formation alone might be responsible for
the NFkB pathway inhibition have failed. Although
one might choose the kinetic parameters to
completely block all IKK in the complexes, this
would subsequently result in a prolonged inhibition,
not observed experimentally. Therefore, in addition
to this process, other mechanisms had to be
proposed.
First, the temperature-dpendent mechanism of
nuclear import had to be introduced. Without it, one
could not capture nuclear accumulation of
constitutive HSP, when in a normal state most of it
should be located in cytoplasm. Since the precise
mechanism behind this transport is not known,
instead of introducing new, unknown molecules that
might mediate this ttansport, we decided to describe
it in the simplest way, introducing the temperature-
dependent transport ration into the equations:
∙
∙
∙
∙
,∙
(1)
∙
∙
∙
∙
∙
∙
,∙
(2)
where k
imp
and k
exp
are nuclear import and nuclear
export rates in normal conditions, k
v
is the ratio of
nuclear and cytoplasmic volumes. Both constitutive
and inducible HSP is assumed to follow that kind of
transport, though the normal rates might differ
between them.
Other than in the model developed by
Szymanska and Zylicz (2009) we wanted to
distinguish between two different outcomes of HSP
acting on the misfolded proteins: through creation of
complexes with them, HSP can either repair them or
target for degradation. Therefore we have created a
pool of generic proteins, both in the nucleus and in
cytoplasm, that are negatively affected by the
temperature and transformed into misfolded
proteins:
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
204
∙
∙
∙
∙
(3)
∙
:
∙
∙
:
∙
∙
∙
(4)
∙
∙
∙
∙
where α represents the efficacy of the repair process
and
is a function dependent on the temperature
as in (Petre et al., 2011):
1
0,4
∙1,4
∙1,45∙
(5)
∙10
8,7∙10
with the last term introduced by us to have
0
(6)
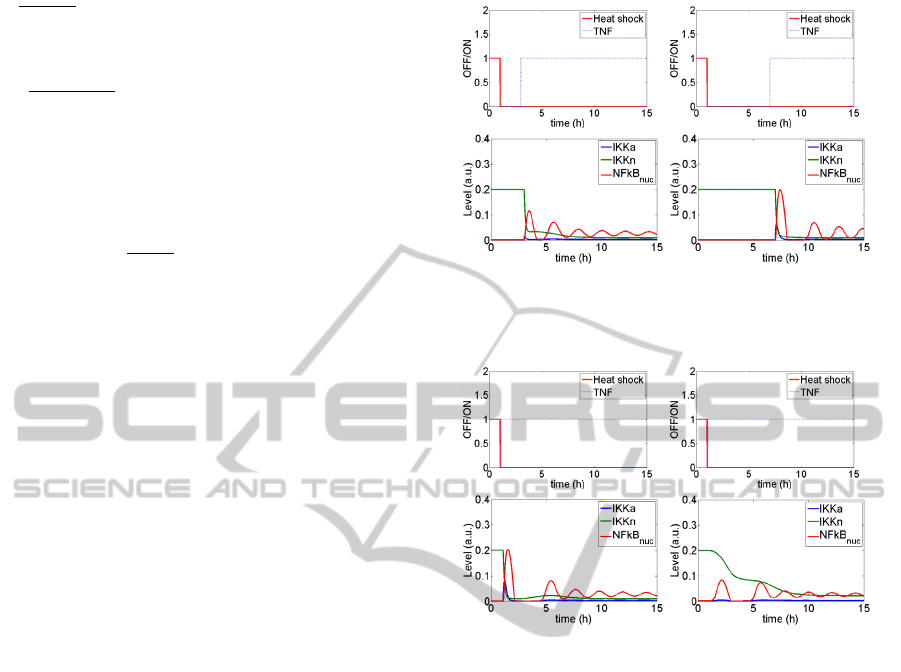
Figure 4 illustrates partial inhibition of the NFkB
pathway in the Model A (Figure 4a) which ceases to
exist if the period between the end of heat shock and
NFkB activation with TNF is too long (Figure 4b).
Despite these promising results, if the TNF
stimulation follows directly heat shock, no such
inhibition is observed (Figure 5a). This is a direct
result of the changes introduced to obtain nuclear
accumulation of constitutive HSP following heat
shock. Since HSP proteins, following their release
from the complexes with HSF1 are transported to
nucleus, too little of them remain in cytoplasm to
effectively bind IKK. Only after inducible form of
HSP is produced as a result of HSF1 pathway, these
newly translated proteins are in place to block NFkB
pathway through IKK inhibition.
As a result, the inhibition of the NFkB pathway
stimulated shortly after the heat shock must be
mediated otherwise than through IKK|HSP
complexes formation. It seems that the inhibition
should be located upstream of IKK activation. One
of the possible explanations is temperature-
dependent misfolding of a kinase activating IKK,
which renders it incapable of such activation. This
assumption is behind the modification introduced in
the model B. Then, inhibition of the NFkB pathway
is visible also in the case when TNF stimulations
follows directly the heat shock (Figure 5b). It is
worth noticing that the frequency of oscillations,
regarded as a specific feature of the NFkB pathway,
remains unchanged even when models of both
pathways are coupled.
a) b)
Figure 4: NFkB pathway responses for two different time
lags between heat shock and TNF stimulation.
a) b)
Figure 5: NFkB pathway response for (a) Model A and (b)
Model B.
5 CONCLUSIONS
This paper presents results of a computational
analysis of a combined HSF1-NFkB pathway. The
model that has been developed captures the
phenomenon of NFkB pathway inhibition through a
heat shock that can last several hours after the heat
shock has ended. The mechanisms behind this
inhibition that have been proposed, i.e. (a) IKK|HSP
complexes creation; (b) temperature-dependent
nuclear import of HSP proteins; (c) temperature-
dependent activation of IKK proteins do not involve
purely theoretical new proteins and it seems that
they are biologically viable. Nevertheless, another
possible explanation could be that the inhibition
takes place at the cell membrane and involves
inactivation of the TNF receptor - a hypothesis that
is currently the subject of experimental
investigation.
InSilicoAnalysisofInteractionsBetweenNFkBandHSFPathways
205

ACKNOWLEDGEMENTS
This work was supported by grants DEC-
2012/05/B/NZ2/01618 (JS, AN, PJ, PW, MKimmel)
and BKM/524/RAU1/2014/t.5 (MKardynska).
REFERENCES
Amman J. U., Haag C., Kasperczyk H., Debatin K. M.,
Fuld S. (2009) Sensitization of neuroblastoma cells for
TRAIL-induced apoptosis by NFkB inhibition, Int. J.
Cancer, 124, 1301–1311.
Ciocca D. R., Calderwood S. K. (2005) Heat shock
proteins in cancer: diagnostic, prognostic, predictive,
and treatment implications. Cell Stress Chaperones
10, 86–103.
Daugaard M., Rohde M, Jaattela M. (2007) The heat
shock protein 70 family: highly homologous proteins
with overlapping and distinct functions. FEBS Lett.
581, 3702–3710.
Domingo-Domènech J., Pippa R., Tápia M., Gascón P.,
Bachs O, Bosch M (2008) Inactivation of NF-κB by
proteasome inhibition contributes to increased
apoptosis induced by histone deacetylase inhibitors in
human breast cancer cells, Breast Cancer Research
and Treatment, 112(1), 53-62.
Fujimoto M., Nakai A. (2010) The heat shock factor
family and adaptation to proteotoxic stress, FEBS
Journal, 277, 4112–4125.
Janus P., Pakula-Cis M., Kalinowska-Herok M., Kashchak
N., Szołtysek K., Pigłowski W., Widlak W., Kimmel
M., Widlak P. (2011), NF-kB signaling pathway is
inhibied by heat shock independently of active
transcription factor HSF1 and increased levels of
inducible heat shock proteins, Genes to Cells, 16,
1168–1175.
Lee Titsworth W., Murad G. J., Hoh B. L., Rahman M
(2014) Fighting fire with fire: the revival of
thermotherapy for gliomas. Anticancer Res. 34(2),
565-574.
Lipniacki T., Paszek P., Brasier A. R., Luxon B., Kimmel
M (2004) Mathematical model of NF-kB regulatory
module. J Theor Biol, 228, 195-215.
Morimoto R. I. (2011) The heat shock response: systems
biology of proteotoxic stress in aging and disease.
Cold Spring Harb Symp Quant Biol. 76, 91-99.
Neznanov N., Komarov A. P., Neznanova L., Stanhope-
Baker P., Gudkov A. V. (2011) Proteotoxic stress
targeted therapy (PSTT): induction of protein
misfolding enhances the antitumor effect of the
proteasome inhibitor bortezomib. Oncotarget 2, 209–
221.
Peper A., Grimbergen C. A., Spaan J. A. E., Souren J. E.
M., van Wijk R. (1997) A mathematical model of the
hsp70 regulation in the cell. Int J Hyperth 14(1), 97–
124.
Petre I., Mizera A., Hyder C. L., Meinander A., Mikhailov
A., Morimoto R. I., Sistonen L., Eriksson J. E., Back
R. (2011) A simple mass-action model for the
eukaryotic heat shock response and its mathematical
validation. Nat. Comp. 10, 595–612.
Ran R., Lu A., Zhang L., Tang Y., Zhu H., Xu H., Feng
Y., Han C., Zhou G., Rigby A. C., Sharp F. R. (2004)
Hsp70 promotes TNF-mediated apoptosis by binding
IKK gamma and impairing NF-kappa B survival
signaling.
Genes Dev. 18, 1466–1481.
Rieger T. R., Morimoto R. I., HatzimanikatisV. (2005)
Mathematical Modeling of the Eukaryotic Heat-Shock
Response: Dynamics of the hsp70 Promoter,
Biophysical Journal, 88, 1646–1658.
Rybinski M., Szymanska Z., Lasota S., Gambin A. (2013)
Modelling the efficacy of hyperthermia treatment, J.
R. Soc. Interface, 10: 20130527.
Sheppard P. W., Sun X., Khammash M., Giffard R. G.
(2014) Overexpression of Heat Shock Protein 72
Attenuates NF-kB Activation Using a Combination of
Regulatory Mechanisms in Microglia. PLoS Comput
Biol 10(2): e1003471.
Sung M. H., Simon R. (2004) In silico simulation of
inhibitor drug effects on nuclear factor-kB pathway
dynamics. Mol. Pharmacol. 66, 70–75.
Szymanska Z., Zylicz M (2009) Mathematical modeling
of heat shock protein synthesis in response to
temperature change, J Theor Biol, 259, 562–569.
Wang Y., Paszek P., Horton C. A., Kell D. B., White M.
R. H., Broomhead DS, Muldoon MR (2011)
Interactions among oscillatory pathways in NF-kappa
B signaling, BMC Sys Biol, 5:23.
Wang Y., Paszek P., Horton C. A., Yue H., White M. R.
H., Kell D. B., Muldoon M. R., Broomhead D. S
(2012) A systematic survey of the response of a model
NF- signalling pathway to stimulation, J Theor Biol,
297, 137-147.
Westerheide S. D, Raynes R., Powell C., Xue B., Uversky
V. N. (2012) HSF transcription factor family, heat
shock response, and protein intrinsic disorder. Curr
Protein Pept Sci. 13(1), 86-103.
Yamamoto Y., Gaynor R. B. (2001) Therapeutic potential
of inhibition of the NF-κB pathway in the treatment of
inflammation and cancer, J Clin Invest. 107(2), 135–
142.
Yang P., Zhou T. (2013) Receptor-dependent sensitivity of
NF-κB to low physiological level, J Biol Sys, 21(3):
1350018.
Zambrano S., Bianchi M. E., Agresti A. (2014), A simple
model of dynamics reproduces experimental
observations, J Theor Biol, 347, 44-53.
Zanotto-Filho A., Braganhol E., Schröder R., de Souza L.
H. T., Dalmolin R. J. S., Bittencourt Pasquali M. A.,
Gelain D. P., Battastini A. M. O., Moreira J. C. F.
(2011) NFκB inhibitors induce cell death in
glioblastomas, Biochemical Pharmacology, 81(3),
412-424.
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
206
