
Amperometric Urea Sensor
Enzyme Immobilization into Adjustable Membrane and Mathematical
Characterization of the Biosensor
Julija Razumiene
1
, Ieva Sakinyte
1
, Vidute Gureviciene
1
and Karolis Petrauskas
2
1
Institute of Biochemistry, Vilnius University, Mokslininku 12, 08662, Vilnius, Lithuania
2
Faculty of Matchematics and Informatics, Vilnius University, Naugarduko 24, Vilnius Lt- 03225, Lithuania
Keywords: Urea Biosensor, Adjustable Membrane, Enzyme, Amperometry, Mathematical Modelling.
Abstract: The prototype of amperometric biosensor for urea determination was designed. The enzyme electrode, made
of a specially developed modified graphite (MG) paste, was produced by covering the electrode surface
with adjustable membrane containing immobilized urease from Canavalia ensiformis (E.C. 3.5.1.5.). Simple
methodology of urea determination in real time has been proposed. The experimental study and the
mathematical model of the biosensor action have been performed.
1 INTRODUCTION
Rapid and simple determination of urea is very
important in clinical analysis. Generally, abnormal
urea concentration indicates kidney disease. The
main procedure of urea level monitoring for patients
is haemodialysis – blood filtering procedure. Filtered
blood flows back to the patient and the leaked out
dialysate is disposed as waste. Certainly, there are
modern apparatus for blood dialysis using ratio
Kt/V, where K is the dialyzer urea clearance, t is the
treatment time, and V is the patient’s urea
distribution volume, to quantify the dialysis dose
(Jensen et al., 2004). Unfortunately, the parameter
Kt/V is not based on real time measurements. In
fact, it is widely accepted that during 4 hours of
haemodialysis Kt/V reaches value about 1.3 and this
is an indication to finish dialysis procedure.
Actually, urea level in blood is highly affected by
stress, physical activity or nutrition and Kt/V would
be relevant only if urea concentration was known
just before dialysis started. Thus, it is very
promising to determine the concentration in real
time. Definitely, the non-invasive and cost-saving
methods are preferable. Thus, in this study we
propose amperometric detection system allowing
detecting urea in waste product - dialysate at any
time of the haemodialysis procedure.
Though the great number of urea determination
methods are based on photometric or conducto-
metric determination of NH
4+
(Patton and Crouch,
1977; Soldatkin et al., 2014) or using a piezo-
electric sensor (Miglior et al., 2007), their
application for express analysis, and especially in
turbid media, is rather complicated. In this case the
amperometric biosensors are most promising. The
electrochemical approach for rapid detection of urea
have been proposed in (Sant et al., 2011) and also in
our previous work (Razumiene et al., 2013).
The goal of this work was on a base of previous
studies to design the urea analyser prototype. The
core of this device is biosensor using especially
developed modified graphite (MG) electrode and
adjustible membrane containing immobilized urease.
The mathematical model was proposed for
characterisation of biosensor action.
2 EXPERIMENTAL
2.1 Preparation of Membrane
Poly(urethane-urea) (PUU) microparticles from
poly(vinyl alcohol) (PVA) and hexamethylene
diisocyanate (HMDI) were synthesized by one-step
method in dimethyl sulfoxide/water (99/1 vol.%)
solution according to previously described protocol
(Budriene et al., 2007).
.
Initial concentration of PVA
was 0.1 M. Initial molar ratio of PVA and HMDI
was 1.0:5.0.
144
Razumiene J., Sakinyte I., Gureviciene V. and Petrauskas K..
Amperometric Urea Sensor - Enzyme Immobilization into Adjustable Membrane and Mathematical Characterization of the Biosensor.
DOI: 10.5220/0005274501440149
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2015), pages 144-149
ISBN: 978-989-758-071-0
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

Immobilization of urease onto PUU microparticles
was carried out in 0.1 M phosphate buffer solution,
pH 7.2. The mixture of the enzyme, buffer and PUU
carrier (in folowing ratio: 1540 U of urease for 0.5 g
of polymeric carrier) was stirred at 25 °C for 30 min
(immediately after synthesis) and then left at 4 °C
overnight. Next day the immobilized enzyme was
thoroughly washed with buffer and 2 µl of
suspension were droped onto terylene film
agglutinated to the rubber ring.
2.2 Preparation of MG and Biosensor
Modified graphite particles were synthesized from
pristine graphite (Merck KGaA) by oxidizing it with
potassium ferricyanide K
3
[Fe(CN)
6
] in alkaline
media. Titration analysis revealed the presence of
small amount (0.14 – 0.17 mmol/g) of basic surface
functional groups.
It was determined that the MG sample suitable
for biosensor design contains a fine fraction of 63 %
with an average diameter of the graphite particles of
20 nm.
MG powder was mixed with the pasting liquid
consisting of 10 % polyvinyl dichloride in acetone
and used for design of the electrodes.
Aiming to design working electrodes MG mixed
with pasting liquid was extruded by forming tablet
(Voitechovic et al., 2010). The tablet was sealed in a
Teflon tube. Electrodes were washed with bidistilled
water, and dried before use. Working urease-MG
electrode (biosensor) was designed by mechanically
attaching the membrane containing polymeric
carriers with immobilized urease to the surface of
MG. (Fig. 1).
2.3 Amperometric Measurements
Urea measurements were performed using a home-
made analyser equipped with a three-electrode
system comprised of a platinum plate electrode as
auxiliary electrode, a saturated Ag/AgCl electrode as
reference and urease-MG (2 mm diameter) as
working electrode (biosensor) (Fig. 2).
The response of the biosensor to the addition of
substrate was investigated under potentiostatic
conditions at 0.2 V (vs. Ag/AgCl) in a stirred buffer
solution. Phosphate buffer solution, pH 7.2,
containing 1 M of urea or dialysate was used as a
substrate. The specially created program and
Origin Pro 8.0 (free trial version from
http://www.originlab.com, OriginLab Corporation,
US) were used for data analysis.
2.3.1 Measurements in Dialysate
Dialysate was analysed using the developed analyser
comprised with the biosensor. Prior the
measurements analyser was tested with standard 1 M
of urea solution. For each measurement 2, 3, 5, 7
and 10 µl of the solution were added into
electrochemical cell containing of 1 ml of buffer
solution. Taking into account that the concentration
of urea in dialysate during the haemodialysis could
be outside the working range of the biosensor, a
dilution of the samples were necessary prior to
analysis to adjust the sample concentration to the
linear range of the biosensor. For this purpose, the
samples of dialysate were 10 times diluted with
buffer solution and analogous experiments were
carried out by adding probes in the electrochemical
cell.
2.4 Mathematical Model
Mathematical model was built aiming to have a tool
for analysing behaviour of the biosensor, impact of
its parameters and to lower number of required
experiments (Amatore et al., 2006). The biosensor is
considered as a reaction-diffusion system when
defining its mathematical model (Baronas et al.,
2010). Due to the biosensor symmetry, the model is
formulated in the one-dimensional space – a line
segment perpendicular to the active surface of the
biosensor. The model is composed of three layers
representing correspondingly the enzyme layer,
terylene membrane and a Nernst diffusion layer that
forms on the external surface of the terylene
membrane. No diffusion of the urea and its products
is assumed in the layer of the MG paste.
The urea detecting process is modelled as a two-
step reaction. In the first step the urea (S) is detected
by the urease (E) in an enzymatic hydrolysis
reaction with production of intermediate compound
carbamic acid (P) and ammonia (P’),
'.PPS
E
(1)
The carbamic acid is finally electrooxidised (not in
one-step mechanism) with production of ammonia
and carbon dioxide (P’’)
'.'' PPP
e
(2)
Electrons released in this reaction are collected by
the MG electrode and form a biosensor response
current.
Reaction (1) takes place in the thin layer,
between the terylene membrane and the MG
electrode. Kinetics of the urea and the carbamic acid
A B
AmperometricUreaSensor-EnzymeImmobilizationintoAdjustableMembraneandMathematicalCharacterizationofthe
Biosensor
145

are described by the following reaction-diffusion
equations:
,
max
2
2
1
SK
SV
x
S
D
t
S
(3)
,
max
2
2
1
SK
SV
x
P
D
t
P
(4)
where S and P are concentrations of the urea and the
carbamic acid, D
1
is a diffusion coefficient, K
M
stands for the Michaelis constant, V
max
is the
maximal reaction rate, t is a time from the start of
the experiment and x stand for a distance from the
electrode surface. Only mass transfer by diffusion is
considered in the terylene membrane and the
external Nernst layer,
,3,2,,
2
2
2
2
i
x
P
D
t
P
x
S
D
t
S
ii
(5)
where D
i
, i=2, 3 are diffusion coefficients of the
species in the corresponding medium.
The experiments start at a moment (t=0) when
the urea is poured into the buffer solution, although
it is still absent in the biosensor. We also assume
zero concentration of the carbamic acid in the
biosensor at this time.
Boundary conditions are defined for the external
bound of the Nernst diffusion layer and the surface
of the graphite electrode. On the upper boundary of
the Nernst diffusion layer, constant concentration
(S
0
) is assumed for urea and the carbamic acid is
absent,
.0,
33
0
xx
PSS
(6)
On the surface of the MG electrode, non-leakage
condition applies to the urea. Electrode oxidation of
the carbamic acid takes place on the surface of the
electrode, thus, a gradient of the carbamic acid is
considered to be equal to the rate of the oxidation
process,
,,0
2
0
1
0
R
x
P
D
x
S
xx
(7)
where R2 stands for the rate of the oxidation
process:
,
0
12
x
PkR
(8)
where k is a heterogeneous oxidation rate constant
and γ is a rate of the active surface of the electrode
to its area.
Response of the biosensor is derived from the
current, produced the oxidation of the carbamic acid
on the surface of the MG electrode. Response
current at a time t is defined as:
,0,)(
2
tFRAnti
e
(9)
where A is an area of the active surface of the
biosensor, n
e
is a number of electrons exchanged in
one reaction event and F stands for the Faraday
constant.
The proposed model consists of a system of non-
linear partial differential equations. Analytical
solutions for such systems are known only for
separate cases, therefore a numerical model was
derived and results obtained by performing
computer simulations.
MG paste is not represented in this model
explicitly. It was assumed, that the MG paste
increases active surface of the electrode and its
impact can be modelled by increasing γ – ratio of the
active electrode surface to its area.
3 RESULTS
3.1 Principle of Urea Biosensor
The urease-MG electrode (biosensor) is illustrated in
Figure 1. The biosensor consists of a Teflon tube (6)
with sealed tablet of MG (4), contact zone for MG
(5), contact wire (7) and adjustable membrane
comprising of immobilized enzyme (3),
semipermeable film (2) and rubber ring (1). The
amperometric urea detection principle is based on
registration of oxidation current observed during the
enzymatic reaction of the intermediate product in
urease-catalyzed hydrolysis of urea (Laurinavicius et
al., 2013).
Figure 1: Principal scheme of urea biosensor.
The biosensor incorporated in to the three-
electrode electrochemical cell is the core of
proposed urea analyser.
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
146
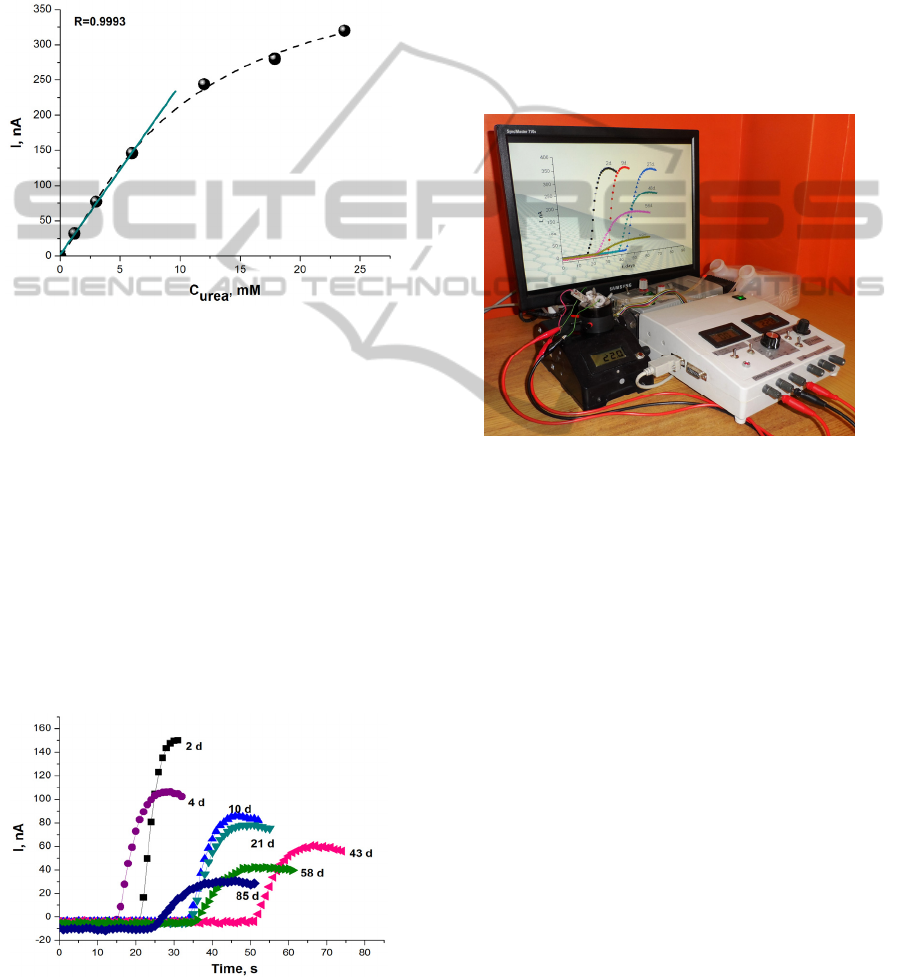
3.2 Characterization of Urea Biosensor
Biosensor based on the urease-MG electrode after
addition of urea in to electrochemical cell shows
substrate-dependent anodic response. The biosensor
response is fast (90 % of steady state current
achieved in 10 s) and this feature is desirable for
analytical instruments. The urea calibration curve
and the linear range are presented in Figure 2.
Figure 2: The urea calibration curve and the linear range
(solid line). Applied urese-MG electrode potential 0.2 V,
Phosphate buffer solution, pH 7.2.
While the linear range of urea biosensor is up to 6
mM the concentration of urea in dialysate during the
haemodialysis will be outside the working range of
the biosensor. Thus, for adjusting the sample
concentration to the linear range of the biosensor a
dilution of the samples were necessary prior to
analysis and the sensitivity of the sensor allows it.
3.3 Urea Biosensor Stability
Stability of the biosensor designed using MG and
membrane containing immobilized urease was
investigated during 85 days (Fig. 3). The responses
Figure 3: Intensity of responses to 3 mM of urea obtained
by proposed analyser at 2 – 85 day.
to the standard urea solution (3 mM) were
periodically recorded at 20 °C and it was detected
that the residual response of the biosensor was not
less than about 50 % of initial magnitude over the
period of 20 days. After 85 days the biosensors
activity decreased up to 20 % of residual.
As can be seen in Figure 3, not only intensity of
responses but also the shape was changed. A
physical explanation and a digital modelling of this
ageing process are following.
3.4 Urea Analyser
The proposed urea analyser is shown in Figure 4.
Figure 4: The prototype of urea analyser based on
amperometric biosensor.
The urea measuring system consists of
electrochemical three-electrode cell, a home-made
potentiostat, peristaltic pump, stirrer and thermostat
and response recorder.
3.5 Urea Determination in Dialysate
Amperometric type of sensors beside other well-
known advantages such as comparable instrumental
sensitivity and amenability to miniaturization also
have one of very important feature – acceptability
for functioning in turbid media. Thus, in this report,
we present simple approach of the biosensor for
determination of urea in dialysate. The
measurements have been carried out by investigating
dialysate leaked out from patients during
haemodialysis. The samples were taken each hour of
blood filtration procedure and in parallel they were
examined at the hospital laboratory. Urea
concentration data for two patients obtained by both
methods are presented in Figure 5.
AmperometricUreaSensor-EnzymeImmobilizationintoAdjustableMembraneandMathematicalCharacterizationofthe
Biosensor
147
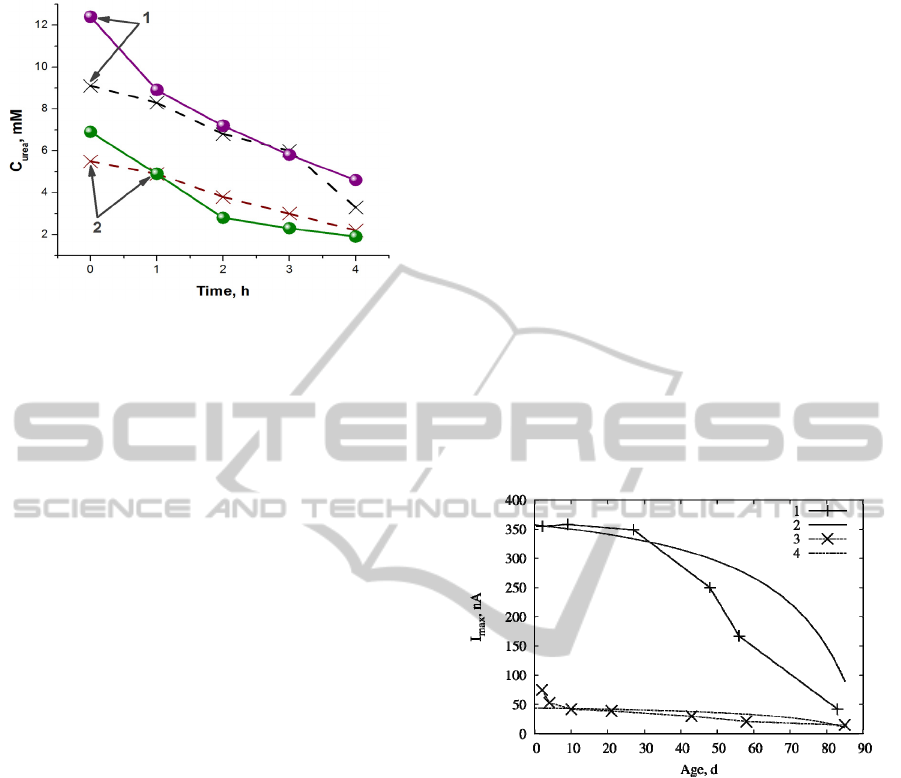
Figure 5: Comparison of urea concentration of two
patients (1 and 2) obtained in dialysate using proposed
analyser (solid line) and in the hospital laboratory (dashed
line).
As can be seen in Figure 5, it was observed good
correlation between data obtained by using analyser
and in the hospital laboratory.
3.6 Computation
Numerical simulations were performed in order to
validate the model, to investigate the biosensor
ageing process as well as impact of the MG paste.
The following parameter values were used in all the
simulations:
,150,12,5
23121
mxxxxx
(10)
,107.3,105.1
1210
2
10
1
smDD
(11)
,3,107.6
0
1210
3
mMSsmD
(12)
,25,5.1
1
max
mMKmMsV
(13)
.3
2
mmA
(14)
The heterogeneous oxidation rate constant k was
not known during this investigation therefore the
product γ×k was used as a single parameter when
performing numerical simulations.
The proposed model does not consider the
decrease of a biosensor response. It will be analysed
in the further investigation. In order to compare
simulation results with the experimental ones,
maximal response current of the experiment was
used.
Initial simulations were performed in order to
characterise the biosensor ageing process and impact
of the MG paste on its response. For the first
attempt, an assumption was made, that the
inactivation of the urease decreases linearly over the
time,
,/)(
maxmax0max
TTTekV
cat
(15)
where T is an age of the biosensor, T
max
is assumed
to be time, during which the enzyme is inactivated
completely, k
cat
= 10
4
s
-1
is the catalytical constant of
the urease, e
0
is the effective concentration of the
urease in the layer between the electrode and the
terylene membrane. Its value was theoretically
estimated to be e
0
< 0.02 mM and derived by fitting
simulation results to be 0.01 mM. Dependence of the
biosensor response on the ageing is shown in Figure
6.
As can be seen in Figure 6, simulated results are
close to the experimentally obtained values only in
some cases. The results show, that increase of the
active area of the electrode surface can have similar
impact as the addition of the MG paste to the
response of the biosensor, although further
investigation is needed to check, if the impact
remains the same at different urea concentrations
and other parameter variations.
Figure 6: Dependence of the biosensor response on the age
on the biosensor. Curves (1, 3) represent experimental
measurements and (2, 4) stand for simulations for the
biosensor with addition of MG paste (1, 2) and without it
(3, 4).
The simulations show, that the inactivation of the
biosensor is not linear as the simulated results do not
fit with the simulations when changing biosensor’s
age. Exponential inactivation rate was also
considered, although no close fit with experimental
data was found.
4 CONCLUSIONS
The biosensor comprised of especially devoted
electrode material MG and adjustable membrane
containing immobilized urease can be applied for
urea analyser.
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
148

Good data correlation with certified method
confirmed that the proposed analyser can be used for
rapid and simple detection of urea in dialysate.
Besides proposed application, the focus of our future
research will be adjustment of the analyser for
veterinary or environment.
Preliminary simulations show, that the sensitivity
of the urea biosensor can be increased by applying
the MG paste to the urease-MG electrode.
ACKNOWLEDGEMENTS
This work was funded by the European Social Fund
under National Integrated Programme Biotechno-
logy and Biopharmacy, grant VP1-3.1-SMM- 08-
K01-005.
REFERENCES
Amatore, C., Oleinick, A. Svir, I., da Mota, N., Thouin, L.,
2006. Theoretical modeling and optimization of the
detection performance: A new concept for
electrochemical detection of proteins in microfluidic
channels, Nonlinear Anal. Model. Control, 11, pp.
345–365.
Baronas, R., Ivanauskas, F., Kulys, J 2010. Mathematical
Modeling of Biosensors; Springer Series on Chemical
Sensors and Biosensors, Springer: Dordrecht.
Netherlands, vol 9.
Budriene, S., Romaskevic, T., Pielichowski, K.,
Pielichowski, J., 2007. Synthesis and characterization
of polyurethane microspheres and their application for
immobilization of maltogenase. Polym. Adv. Technol.,
vol. 18, no. 1, pp. 67-71.
Jensen, P. S., Bak, J., Ladefoged, S., Andersson-Engels,
S., Friis-Hansen, L., 2004. Online monitoring of urea
concentration in dialysate with dual-beam Fourier-
transform near-infrared spectroscopy. Journal of
Biomedical Optics, vol. 9, no. 3, pp. 553-557.
Laurinavicius, V., Razumiene, J., Gureviciene, V., 2013.
Bioelectrochemical conversion of urea on Carbon
Black Electrode and Application. IEEE Sensors
Journal, vol. 13, no. 6, pp. 2208-2213.
Miglior, F., Sewalem, A., Jamrozik, J,. Bohmanova J.,
Lefebre, D.M., Motore, R.K., 2007. Genetic Analysis
of Milk Urea Nitrogen and Lactose and Their
Relationships with Other Production Traits in
Canadian Holstein Cattle. J. Dairy Sc., vol. 90, no. 5,
pp. 2468-2479.
Patton, C.J., Crouch,. S.R., 1977. Spectrophotometric and
kinetics investigation of the Berthelot reaction for the
determination of ammonia. Anal. Chem., vol. 49, no.
3, pp. 464-469.
Razumiene, J., Sakinyte, I. Kochane, T. Maciulyte, S.,
Straksys, A., Budriene, S., Barkauskas J., 2013.
Carbon Electrode based Urea Sensor - Modification of
Graphite and New Polymeric Carriers for Enzyme
Immobilization. Proceedings of the BIODEVICES
2013, 6
th
International Conference on Biomedical
Electronics and Devices, pp. 197-201. Available from:
SCITEPRESS.
Sant, W., Temple-Boyer, P., Chanié, E., Launay, J.,
Martinez, A. 2011. On-line monitoring of urea using
enzymatic field effect transistors. Sensors and
Actuators B: Chemical, vol. 160, no. 1, pp. 59-64.
Soldatkin, O. O., Kucherenko, I.S., Marchenko, S. V.,
Ozansoy Kasap, B., Akata, B., Soldatkin, A. P.,
Dzyadevych, S. V., 2014. Application of
enzyme/zeolite sensor for urea analysis in serum.
Materials Science and Engineering: C, vol., no. 42,
pp. 155-160.
Voitechovic, E., Razumiene, J., Sakinyte, E., Barkauskas,
J., 2010. Investigation of bioelectrocatalytic systems
with PQQ-dependent GDH and carbonaceous
materials. Biologija, vol. 56, no.1-4, pp. 83-87.
AmperometricUreaSensor-EnzymeImmobilizationintoAdjustableMembraneandMathematicalCharacterizationofthe
Biosensor
149
